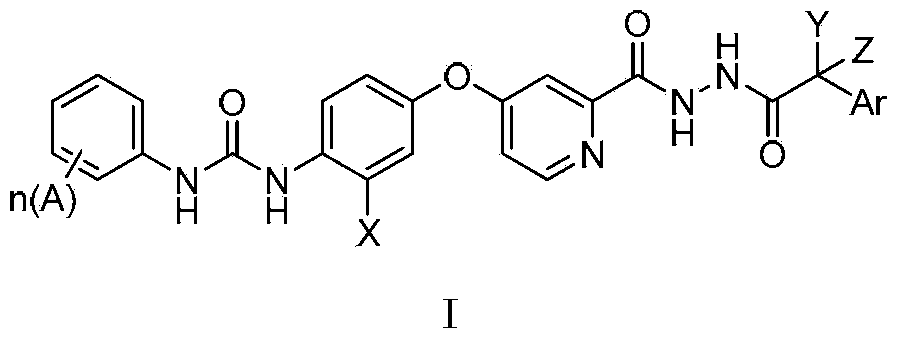
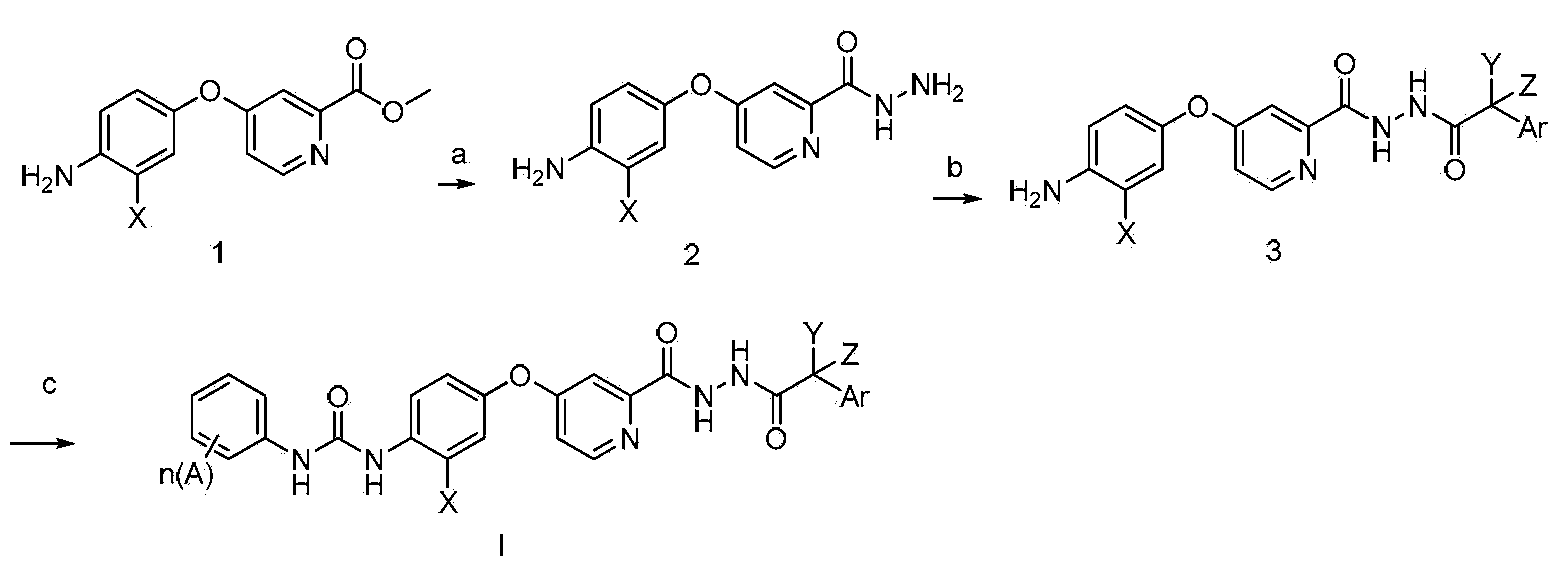
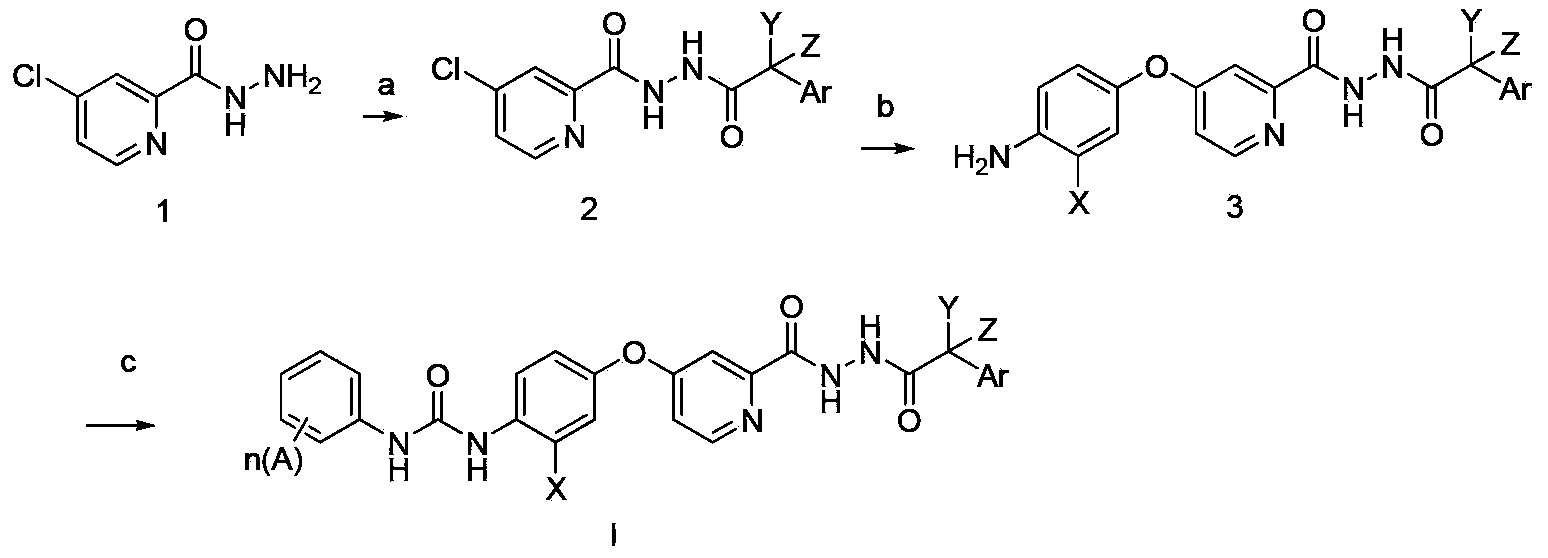
N'-aryl acetyl o-pyridine hydrazide derivatives, and preparation methods, pharmaceutical composition and application thereof
A kind of technology of pyridine hydrazide and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1.1-(4-(2-(2-(3-fluorophenylacetyl)hydrazinecarbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-trifluoromethylphenyl) Urea
[0076]
[0077] Synthesis of 4-(p-aminophenoxy)pyridine-2-hydrazide
[0078] Add 1.6g of methyl 4-(p-aminophenoxy)pyridine-2-carboxylate into 10mL of methanol, add 0.5mL of hydrazine hydrate dropwise at 0°C, after the addition is complete, move to room temperature and react for 18 hours. TLC monitors that the reaction is complete , most of the solvent was distilled off under reduced pressure, filtered, and the filter cake was washed 3 times with methanol to obtain 1.4 g of off-white solid. 1 HNMR (300MHz, DMSO-d 6 ):9.84(s,1H,-CONH-),8.43(d,1H,Ar-H),7.30(s,1H,Ar-H),7.04(m,1H,Ar-H),6.88(s, 1H,Ar-H),6.85(s,1H,Ar-H),6.66(s,1H,Ar-H),6.63(s,1H,Ar-H),5.18(s,2H,Ar-NH 2 ),4.52(br,2H,CONH-NH 2 ).MS(FAB):(M + +1=245).
[0079] Synthesis of N'-m-fluorophenylacetyl-4-(p-aminophenoxy)pyridine-2-hydrazide
[0080] Dissolve 105mg (0.43mmol) of com...
Embodiment 2
[0086] Example 2.1-(4-(2-(2-(3-fluorophenylacetyl)hydrazinecarbonyl)pyridine-4-oxyl)o-fluorophenyl)-3-(4-chloro-3-trifluoromethylbenzene base) urea
[0087]
[0088] Utilize 4-(p-amino-m-fluorophenoxy)pyridine-2-methyl carboxylate to replace 4-(p-aminophenoxy)pyridine-2-methyl carboxylate, carry out with reference to the operation of Example 1, and obtain the target compound as white Solid 135mg. 1 H NMR (300MHz, DMSO-d 6 ):10.56(br,1H,-CONH-),10.30(br,1H,-CONH-),9.57(s,1H,-CONH-),8.77(s,1H,-CONH-),8.56(d, 1H,Ar-H),8.16(m,2H,Ar-H),7.63(s,2H,Ar-H),7.37(m,3H,Ar-H),7.17(m,5H,Ar-H) ,3.56(s,2H,-CH 2 -Ar).MS(FAB)(M + +1=620)
Embodiment 3
[0089] Example 3.1-(4-(2-(2-(3,5-dichlorophenylacetyl)hydrazine carbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-trifluoromethyl Phenyl)urea
[0090]
[0091] Using 3,5-dichlorophenylacetic acid instead of m-fluorophenylacetic acid, the operation was carried out with reference to Example 1, and 135 mg of the target compound was obtained as a white solid. 1 H NMR (400MHz, DMSO-d 6 ): 10.53(s,1H,-CONH-),10.34(br,1H,-CONH-),9.37(s,1H,-CONH-),9.14(s,1H,-CONH-),8.54(s, 1H,Ar-H),8.12(s,1H,Ar-H),7.60(m,4H,Ar-H),7.38(s,1H,Ar-H),7.06(m,6H,Ar-H) ,3.57(s,2H,-CH 2 -).MS(FAB)(M + +1=653)
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com