Ambrisentan degradation product and preparation method thereof
A degradation product, ambrisentan technology, applied in the field of biomedicine, can solve the problems of undisclosed degradation products, unknown structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
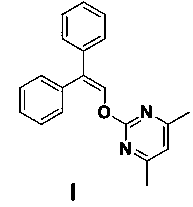
[0012] Example 1 Preparation of ambrisentan degradation product I
[0013]
[0014] Sodium hydride (3.5 g, 0.08 mol) was added to 50 mL of DMF solution, under nitrogen protection, cooled to 0~5 degrees, and then 2,2-diphenylacetaldehyde (5 g, 0.026 mol ) in DMF was added dropwise (50 mL) solution, maintain the temperature at 0~5 degrees, continue to stir the reaction for 0.5 h after the drop, and then add dropwise 4,6-dimethyl-2-methylsulfonylpyrimidine (5.2 g, 0.028 mol ) in DMF (50 mL) solution, continue to stir for 2 h after dripping, TLC detects that the reaction is complete, pour the reaction solution into 200 mL of ice-water mixture, filter, wash the filter cake with water (30 ml x 3) for 3 times, and then vacuum-dry to obtain a 8 g of white solid is the degradation product I of ambrisentan.
[0015] Mass Spectrum (ESI): m / z 303.1 (M+H) + .
[0016] 1 H NMR (400 MHz, CDCl 3 ) δ: 7.91 (s, 1H), 7.43 (d, J = 8 Hz, 2H), 7.35 ~ 7.25 (m, 8H), 6.78 (s, 1H), 2.44 (s, 6...
Embodiment 2
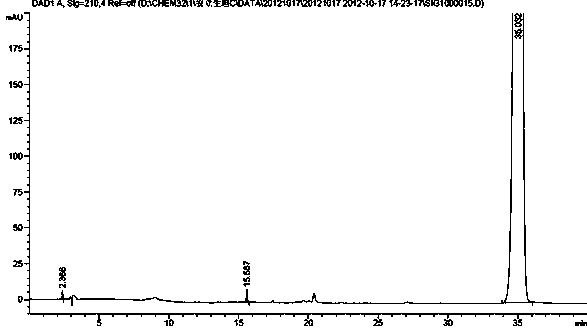
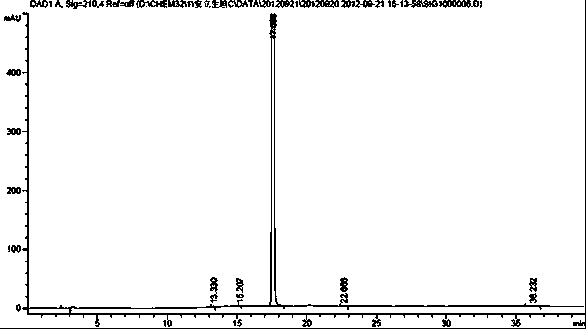
[0018] Example 2 Separation and detection of ambrisentan degradation product I
[0019] HPLC conditions:
[0020] Octadecylsilane-bonded silica gel was used as filler, mobile phase A was 0.01 mol / L dipotassium hydrogen phosphate buffer (phosphoric acid adjusted to pH 4.5), mobile phase B was acetonitrile, and the gradient elution was as follows Table 1 For elution, the detection wavelength is 210 nm, and the column temperature is 30 °C. The theoretical plate number should not be less than 5000 based on the calculation of Anlishengtan peak.
[0021] Gradient elution table 1
[0022] time (min) Mobile phase A (%) Mobile phase B (%) 0 90 10 3 90 10 13 35 65 40 35 65
[0023] Take an appropriate amount of impurity I and ambrisentan respectively, dilute with acetonitrile to prepare 0.5 mg / mL, inject 20 μL each into the sample, and separate and detect under the above HPLC conditions.
[0024] Analysis results: The HPLC chart of Ambrisent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com