A kind of ambrisentan degradation product and preparation method thereof
A compound and selected technology, applied in the field of biomedicine, can solve the problems of undisclosed degradation products, unknown structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
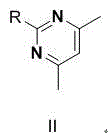
[0012] Embodiment 1 Preparation of Ambrisentan Degradation Product I
[0013]
[0014] Sodium hydride (3.5g, 0.08mol) was added to 50mL of DMF solution, under nitrogen protection, cooled to 0~5 degrees, then 2,2-diphenylacetaldehyde (5g, 0.026mol) in DMF (50mL ) solution, maintain the temperature at 0~5 degrees, continue to stir the reaction for 0.5h after dropping, then add dropwise a DMF (50mL) solution of 4,6-dimethyl-2-methylsulfonylpyrimidine (5.2g, 0.028mol), Continue to stir for 2 hours after dropping, TLC detects that the reaction is complete, pour the reaction solution into 200mL of ice-water mixture, filter, wash the filter cake with water (30mlx3) 3 times, and then vacuum-dry to obtain 8g of off-white solid, which is ambrisentan The degradation product I.
[0015] Mass spectrum (ESI): m / z303.1 (M+H) + .
[0016] 1 HNMR (400MHz, CDCl 3 )δ:7.91(s,1H),7.43(d, J =8Hz,2H),7.35~7.25(m,8H),6.78(s,1H),2.44(s,6H).
[0017] 13 CNMR (101MHz, CDCl 3 )δ: 169.16, 162....
Embodiment 2
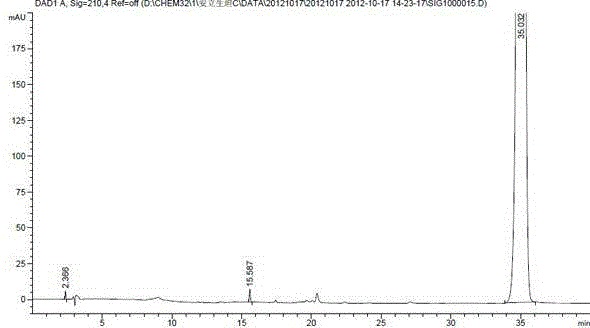
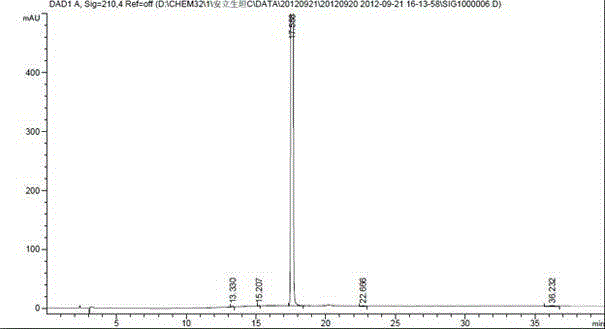
[0018] Example 2 Separation and Detection of Ambrisentan Degradation Product I
[0019] HPLC conditions:
[0020] Octadecylsilane bonded silica gel was used as filler, mobile phase A was 0.01mol / L dipotassium hydrogen phosphate buffer (phosphoric acid adjusted to pH 4.5), mobile phase B was acetonitrile, and the gradient elution was as follows Table 1 For elution, the detection wavelength is 210nm, and the column temperature is 30°C. The theoretical plate number should not be less than 5000 based on the calculation of Anlishengtan peak.
[0021] Gradient elution table 1
[0022] time (min) Mobile phase A (%) Mobile phase B (%) 0 90 10 3 90 10 13 35 65 40 35 65
[0023] Take an appropriate amount of impurity I and ambrisentan respectively, dilute with acetonitrile to prepare 0.5 mg / mL, inject 20 μL into the sample, and separate and detect under the above HPLC conditions.
[0024] Analysis results: The HPLC chart of Ambrisentan Impur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com