A kind of lithium ion battery gradient cathode material precursor and preparation method thereof
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of low bulk density, difficulty in achieving uniform distribution of transition metal elements, and hindering the practical application of materials, and achieve narrow particle size distribution , high density and good sphericity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
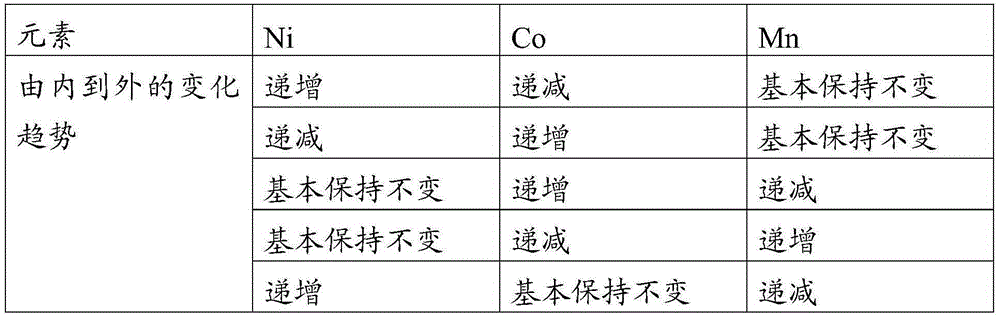
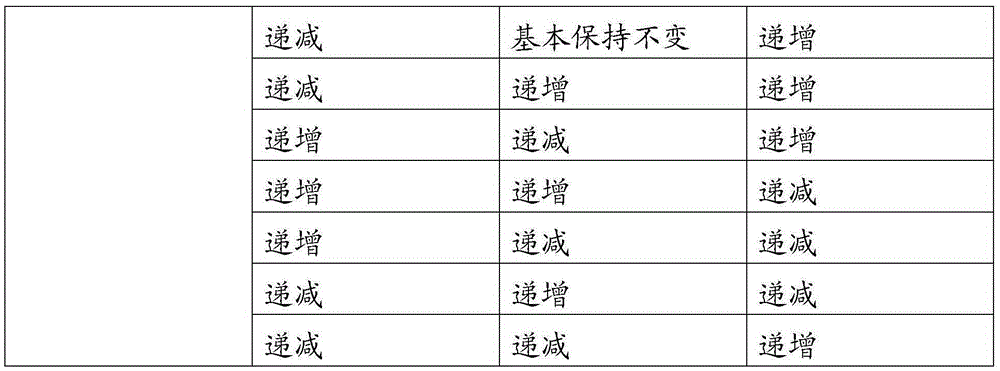
[0036] NiSO 4 , CoSO 4 , MnSO 4 The molar ratios are respectively Ni:Co:Mn=0.5:0.2:0.3 and 1 / 3:1 / 3:1 / 3 to prepare solutions A and B. The volumes of solutions A and B are the same and the total concentration is 1.5mol / L, prepare 5.0mol / L sodium hydroxide solution, prepare ammonia solution with a concentration of 10.0mol / L, add solution A to solution B at a speed of 50L / h with a metering pump, and stir evenly at the same time at a speed of 100L / h Flow into the reactor, at the same time, sodium hydroxide solution and ammonia solution are also added to the reactor for reaction. Under the condition of nitrogen protection, the temperature of the reaction vessel was controlled at 50° C., the pH value was 11.0, the stirring speed was 500 r / min, and the flow rate of ammonia water was 15 L / h. There is no need to overflow during the reaction process, and the reaction can be stopped after the consumption of solutions A and B at the same time. Solid-liquid separation was carried out a...
Embodiment 2
[0038] NiCl 2 , CoCl 2 , MnCl 2 Prepare solutions A and B at the ratio of Ni:Co:Mn=0.4:0.3:0.3 and 0.8:0.1:0.1 respectively by molar ratio. The volume of solutions A and B is the same and the total concentration is 2.0mol / L. Prepare 5.0mol / L Sodium hydroxide solution, prepare ammonia solution with a concentration of 8.0mol / L, use a metering pump to add solution A to solution B at a speed of 100L / h, stir evenly and then flow into the reaction kettle at a speed of 200L / h, and At the same time, sodium hydroxide solution and ammonia solution are also added to the reactor for reaction. Under the protection condition of argon, the temperature of the reaction vessel was controlled at 45° C., the pH value was 11.5, the stirring speed was 300 r / min, and the flow rate of ammonia water was 25 L / h. There is no need to overflow during the reaction process, and the reaction can be stopped after the consumption of solutions A and B at the same time. Solid-liquid separation was carried ou...
Embodiment 3
[0040] NiSO 4 , CoSO 4 , MnSO 4 Solution A is prepared in a molar ratio of Ni:Co:Mn=0.5:0.2:0.3, Ni(NO 3 ) 2 , Co(NO 3 ) 2 , Mn(NO 3 ) 2 Prepare solution B in the ratio of Ni:Co:Mn=0.2:0.2:0.6 in molar ratio, the volume of solution A and B is the same and the total concentration is 1.5mol / L and 2.0mol / L respectively, prepare 4.0mol / L of hydroxide Potassium solution, prepare a citric acid solution with a concentration of 15.0mol / L, add solution A to solution B at a speed of 80L / h with a metering pump, and flow into the reaction kettle at a speed of 160L / h after stirring evenly. , Sodium hydroxide solution and ammonia solution are also added to the reaction kettle for reaction. Under nitrogen protection conditions, the temperature of the reaction vessel was controlled at 60° C., the pH value was 12.0, the stirring speed was 800 r / min, and the flow rate of the citric acid solution was 20 L / h. There is no need to overflow during the reaction process, and the reaction can ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bulk density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com