Medical application of berberine derivative
A berberine and drug technology, which is applied in the field of berberine derivatives, can solve the problems of unseen glioma migration and invasion, and unsatisfactory drug efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
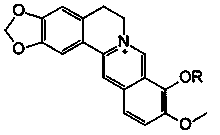
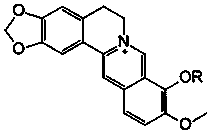
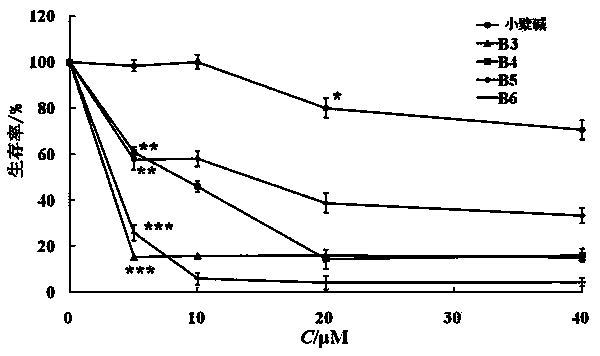
[0030] Synthesis and Identification of Berberine Derivatives
[0031]Berberine hydrochloride was purchased from Xi’an Xiaocao Plant Technology Co., Ltd., with a purity of 97%; bromododecane, bromohexadecane, bromooctadecane, and benzyl bromide were all analytically pure, purchased from Aladdin Reagent Co., Ltd. Company; N, N-dimethylformamide (DMF) is analytically pure, dried with molecular sieves, and purchased from Tianjin Fuyu Fine Chemical Co., Ltd.; neutral alumina for chromatography is a product of Sinopharm Group; DMEM medium (R10 -013-cv), fetal bovine serum (FBS), trypsin, penicillin and streptomycin were all purchased from Cellgro, USA; tetramethylazozolium salt (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma Company of the United States; 4% paraformaldehyde was purchased from Guangzhou Jingxin Technology Co., Ltd.; the experimental water was ultrapure water. Rat glioma C6 cells and human glioma U87 cells were from the ATCC cell bank in the United St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com