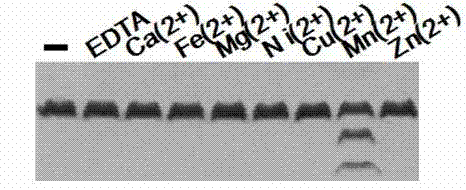Enzyme activity starting and improving method of Deinococcus radiodurans protease PprI
A technology of Deinococcus radiodurans and protease, applied in the direction of microorganism-based methods, biochemical equipment and methods, hydrolytic enzymes, etc., can solve undiscovered problems, achieve the effect of improving enzyme cutting activity and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) The protease activity of PprI depends on the metal ion Mn 2+
[0031] When PprI protein performs protease activity, it needs metal ion Mn 2+ The presence. in Mn 2+ The activity is optimal when the final concentration is 2mM / L.
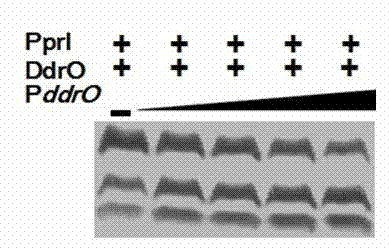
[0032] (2) Restriction substrate gene wxya The promoter can promote the efficiency of PprI digestion
[0033] Digest substrate protein DdrO and substrate gene promoter in reaction buffer (150mM NaCl, 20mM Tris-HCl 8.0, 1mM DTT, 10mM MgCl 2 ) for 40 minutes. Next, the purified PprI protein was added to the reaction solution. Then, add MnCl at a final concentration of 2.0 mM 2 Start the reaction. After 40 minutes, the reaction was terminated and detected by SDS-PAGE electrophoresis. Experiments have shown that the interaction between the substrate protein and its own promoter can greatly promote the efficiency of PprI digestion.
Embodiment 2
[0035] (1) The protease activity of PprI depends on the metal ion Mn 2+
[0036] When PprI protein performs protease activity, it needs metal ion Mn 2+ The presence. in Mn 2+ The activity is optimal when the final concentration is 2mM / L. Other divalent ions such as Ni 2+ ,Zn 2+ etc., the final concentration is greater than 0.25mM / L, all have inhibitory effect on protease activity.
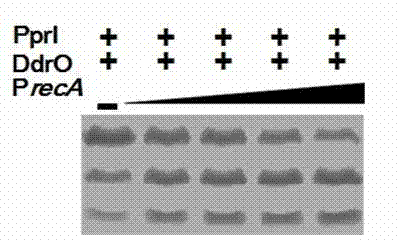
[0037] (2) recA Gene promoter can promote PprI digestion efficiency
[0038] Digestion of substrate protein DdrO with recAGene promoter in reaction buffer (150mM NaCl, 20mM Tris-HCl 8.0, 1mM DTT, 10mM MgCl 2 ) for 40 minutes. Next, the purified PprI protein was added to the reaction solution. Then, add MnCl at a final concentration of 2.0 mM 2 Start the reaction. After 40 minutes, the reaction was terminated and detected by SDS-PAGE electrophoresis. Experiments have shown that substrate proteins and recA The interaction between gene promoters can greatly promote the digestio...
Embodiment 3
[0040] (1) The protease activity of PprI depends on the metal ion Mn 2+
[0041] When PprI protein performs protease activity, it needs metal ion Mn 2+ The presence. in Mn 2+ The activity still exists when the final concentration is 5mM / L. Other divalent ions such as Fe 2+ ,Cu 2+ etc., the final concentration is greater than 0.25mM / L, all have inhibitory effect on protease activity.
[0042] (2) Non-specific DNA cannot promote the digestion efficiency of PprI
[0043] Digest substrate protein DdrO and non-specific DNA in reaction buffer (150mM NaCl, 20mM Tris-HCl 8.0, 1mM DTT, 10mM MgCl 2 ) for 40 minutes. Next, the purified PprI protein was added to the reaction solution. Then, add MnCl at a final concentration of 2.0 mM 2 Start the reaction. After 40 minutes, the reaction was terminated and detected by SDS-PAGE electrophoresis. Experiments have shown that non-specific DNA cannot promote the efficiency of PprI digestion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com