Eucommia ulmoides fine powder and preparation method thereof
A technology of Eucommia ulmoides powder and content, which is applied in food preparation, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problem of hindering the effective absorption of Eucommia active ingredients, increasing the extraction cost of Eucommia active ingredients, and reducing the concentration of Eucommia active ingredients. and other problems, to achieve the effect of clear solution, short time and avoid damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Eucommia fine powder includes the following ingredients: total sugar content 15%, chlorogenic acid content 5%, total flavonoid content 15%, geniposide content 3.5%, aucubin content 0.8%, water content 6.0%, and Trace element B 8μg / g, Ca 4300μg / g, Cu 21μg / g, Mg 3101μg / g, Mn 110μg / g, P 1912μg / g, Se 0.54μg / g, Zn 31μg / g, Na 160μg / g, K3112μg / g, Fe 210 μg / g.
[0028] The preparation method is:
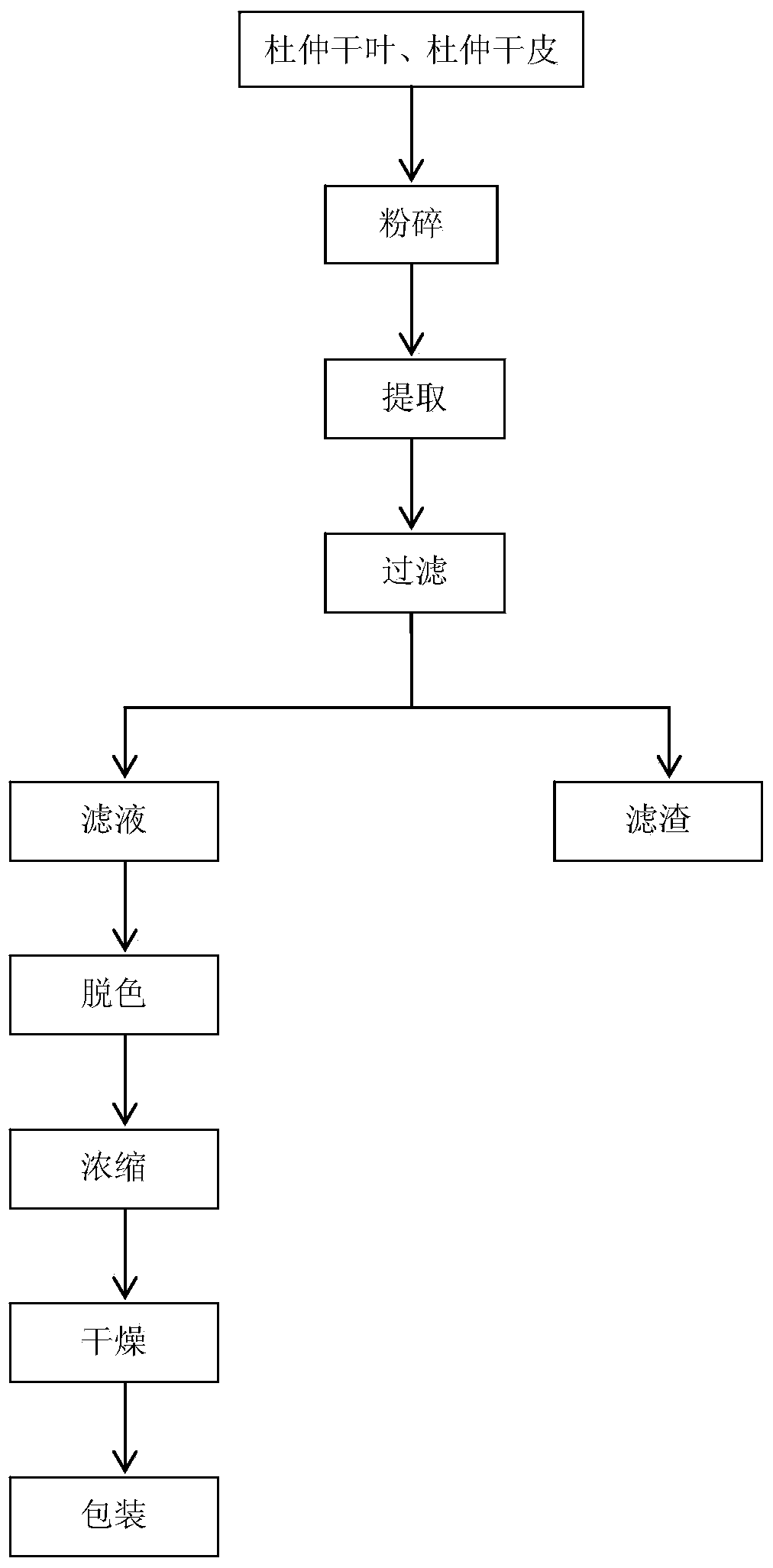
[0029] (1) Pre-treatment: After removing impurities, the dried bark of Eucommia is passed the inspection according to the quality requirements of the Chinese Pharmacopoeia, then exposed to the sun or subjected to high-temperature instant anti-virus drying; the dried bark of Eucommia is mechanically crushed to 10-20 mesh , placed in a dry and cool place for later use.
[0030] (2) Extraction: take the dried bark of Eucommia treated in step (1), use water as the extract, mix according to the ratio of solid to liquid 1:20, extract at 60°C for 3 hours, filter the extract, pump To the ...
Embodiment 2
[0034] Eucommia fine powder includes the following ingredients: total sugar content 18%, chlorogenic acid content 7%, total flavonoid content 18%, geniposide content 1.8%, aucubin content 1.2%, water content 5.0%, and Trace elements B 59μg / g, Ca 190000μg / g, Cu 6.9μg / g, Mg5890μg / g, Mn 179μg / g, P 1650μg / g, Se 0.56μg / g, Zn 35μg / g, Na500μg / g, K 7500μg / g g, Fe 180 μg / g.
[0035] The preparation method is:
[0036] (1) Pre-treatment: After removing impurities, the leaves of Eucommia ulmoides are passed the inspection according to the quality requirements of the Chinese Pharmacopoeia, then exposed to the sun or subjected to high-temperature instant sterilization and drying; the dried leaves of Eucommia are mechanically crushed to 10-20 mesh , placed in a dry and cool place for later use.
[0037] (2) Extraction: take Eucommia leaves or bark treated in step (1), use water as the extract, mix according to the ratio of solid to liquid 1:15, extract at 65°C for 2.5h, and filter the ext...
Embodiment 3
[0041] Eucommia fine powder includes the following ingredients: total sugar content 19%, chlorogenic acid content 15%, total flavonoid content 21%, geniposide content 2.1%, aucubin content 0.9%, water content 6.0%, and Trace elements B 55μg / g, Ca 198000μg / g, Cu 7.12μg / g, Mg5998μg / g, Mn 168μg / g, P 1689μg / g, Se 0.56μg / g, Zn 34.58μg / g, Na478μg / g, K 7489μg / g, Fe 199 μg / g. The preparation method is:
[0042] (1) Pre-treatment: After removing impurities, the leaves of Eucommia ulmoides are passed the inspection according to the quality requirements of the Chinese Pharmacopoeia, then exposed to the sun or subjected to high-temperature instant sterilization and drying; the dried leaves of Eucommia are mechanically crushed to 10-20 mesh , placed in a dry and cool place for later use.
[0043] (2) Extraction: take Eucommia leaves or bark treated in step (1), use water as the extract, mix according to the ratio of solid to liquid 1:5, extract at 70°C for 2 hours, filter the extract, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com