Hydrates of proline derivative salt and production method thereof
A 1.3-{, methyl technology, applied in the field of hydrate of proline derivative salt and its production, can solve the problems of unfavorable drug product development, undiscovered polymorphic crystals, etc.
Active Publication Date: 2014-12-24
NANJING HUAWE MEDICINE TECH DEV
View PDF6 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Therefore, these properties observed in the 3 hydrochloride salt of compound I are considered unfavorable for the development of drug products
[0006] Moreover, although the invention discloses specific salts of Compound I and its thiazolidine derivatives as example compounds, no discussion of polymorphic crystals of the example compounds has been found
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
Login to View More
Abstract
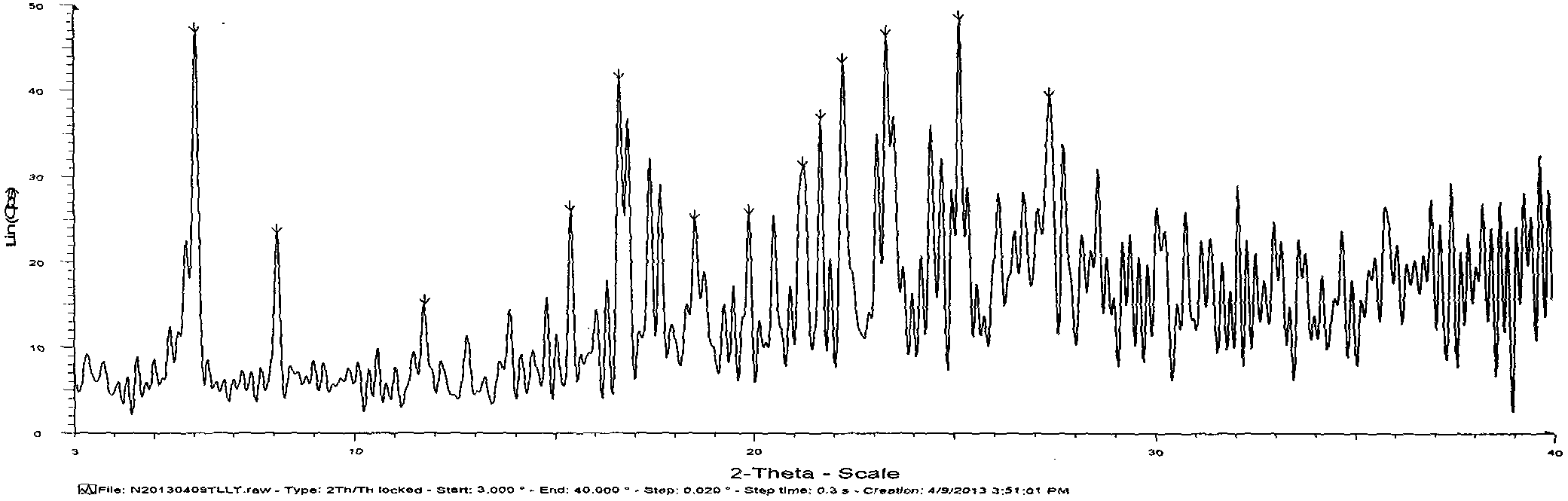
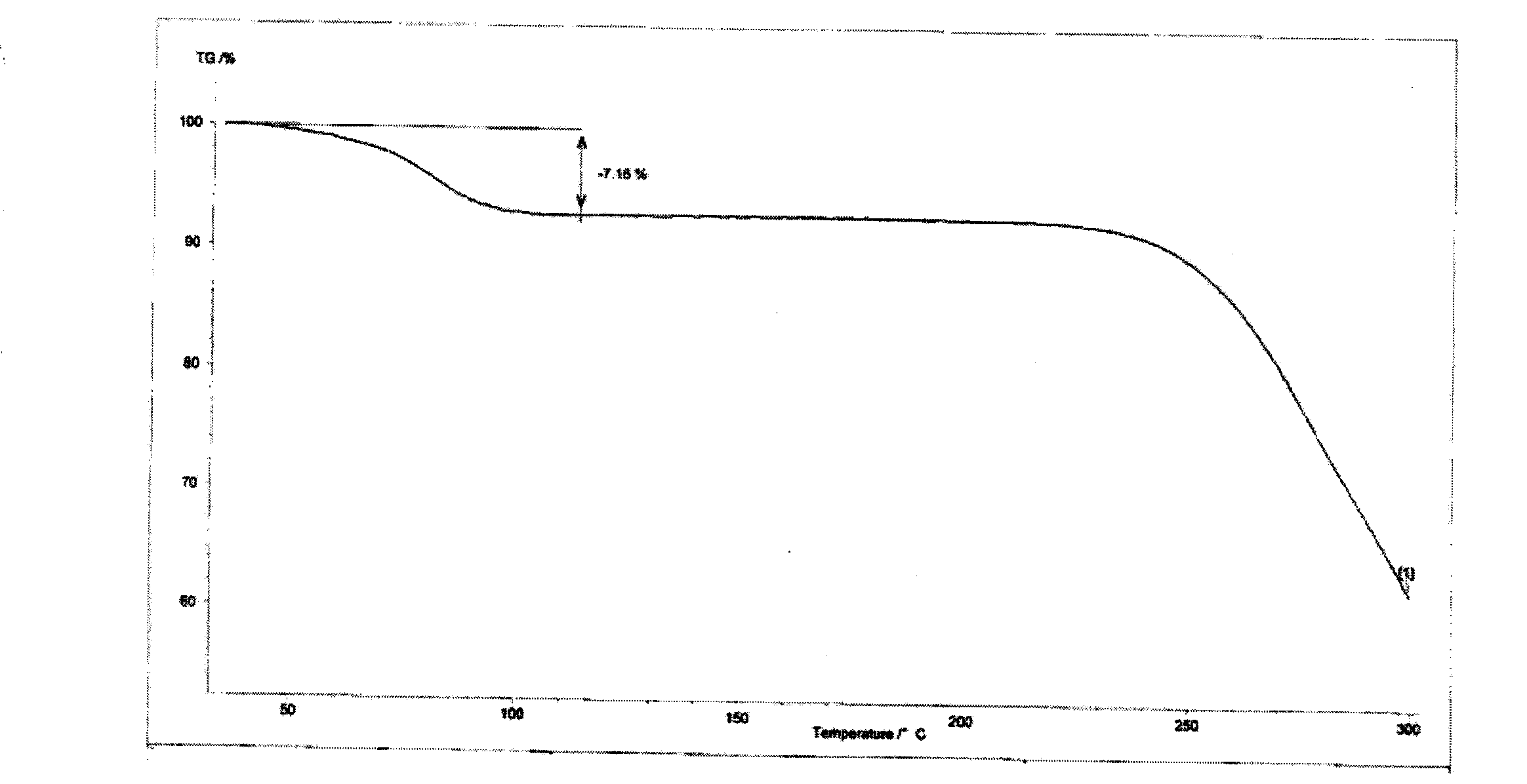
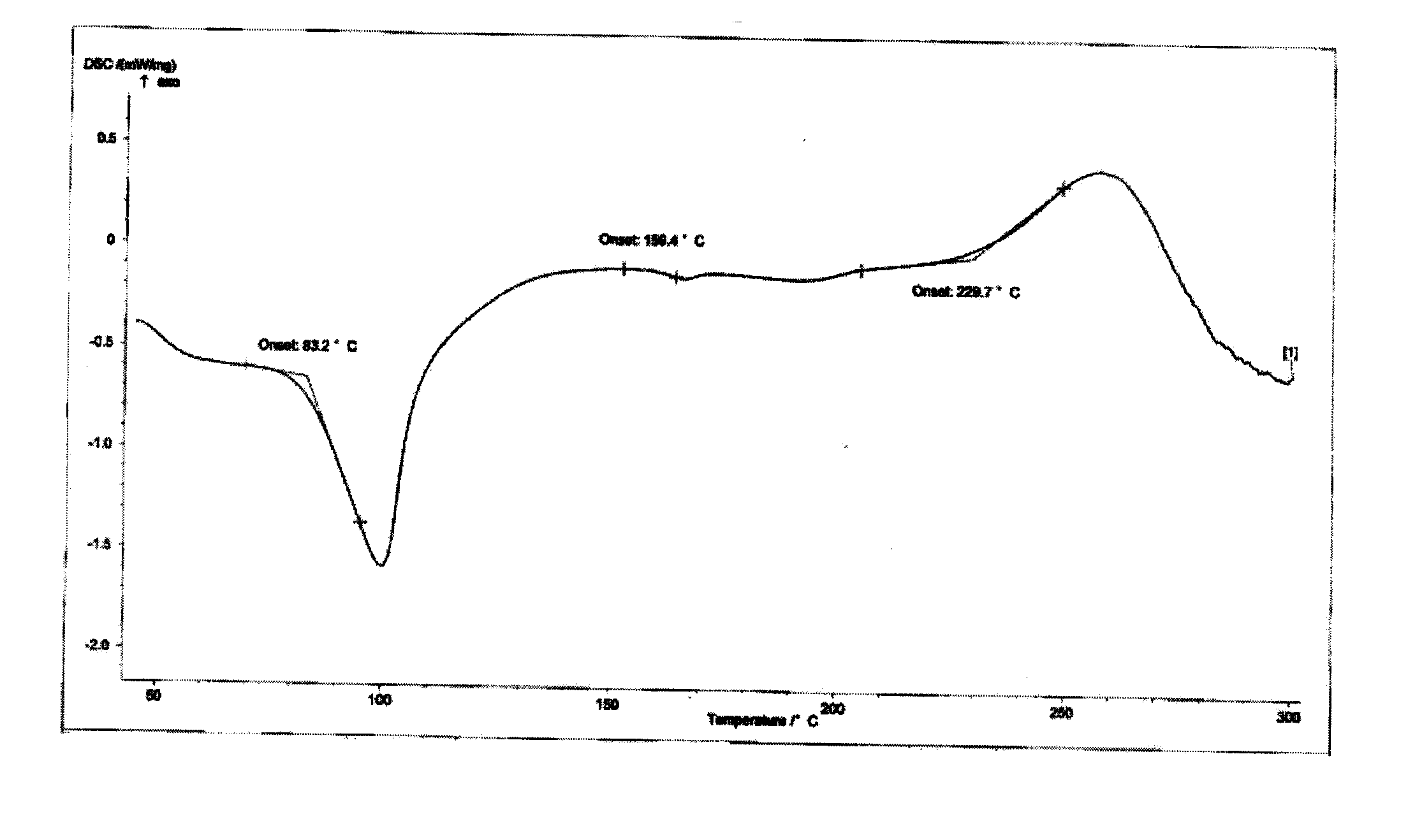
The invention provides hydrates of a proline derivative salt, namely, 2.5-3.5 hydrate crystals of 3-{(2s,4s)-4-4[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidine-2-ylcarbonyl}thiazolidine 2.5 hydrobromide serving as dipeptidyl peptidases (i.e. DPP-IV) inhibitors. The crystals have the advantages of excellent stability and solubility as well as reproducibility and the invention also provides a production method of the crystals.
Description
technical field [0001] The present invention relates to novel 3-{(2s,4s)-4-4[4-(3-methyl-1-phenyl-1H-pyridines) acting as dipeptidylase-IV (i.e. DPP-IV) inhibitors Salts and solvates thereof of oxazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl}thiazolidine, specifically relating to 3-{(2s,4s)-4-4[4-(3 -Methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl}thiazolidine 2.5 hydrobromide 2.5 to 3.5 hydrate. Background technique [0002] DPP-IV is an amino acid sequence that recognizes that the second amino acid from the nitrogen terminal is proline (also can be alanine or hydroxyproline), and generates serine of the dipeptide Xaa-Pro (Xaa represents any amino acid, Pro represents proline). DPP-IV is known to be widely distributed in mammalian tissues, especially in blood, kidney, intestinal epithelium and placenta. [0003] DPP-IV inhibitors inhibit the inactivation of glucagon-like peptide-1 in plasma and potentiate their incretin action. Therefore, they ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D417/14
CPCC07D417/14
Inventor 宋志春胡丹丹蒋玉伟张孝清
Owner NANJING HUAWE MEDICINE TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com