Negative photosensitive composition capable of curing at low temperature
A technology of photosensitive composition and compound, which is applied in the direction of optics, photomechanical equipment, photoplate process of pattern surface, etc., can solve the problems of compromising the storage stability of the composition, and the stability of the low-temperature curing composition has not been found, and achieves High chemical resistance, high environmental tolerance, excellent stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
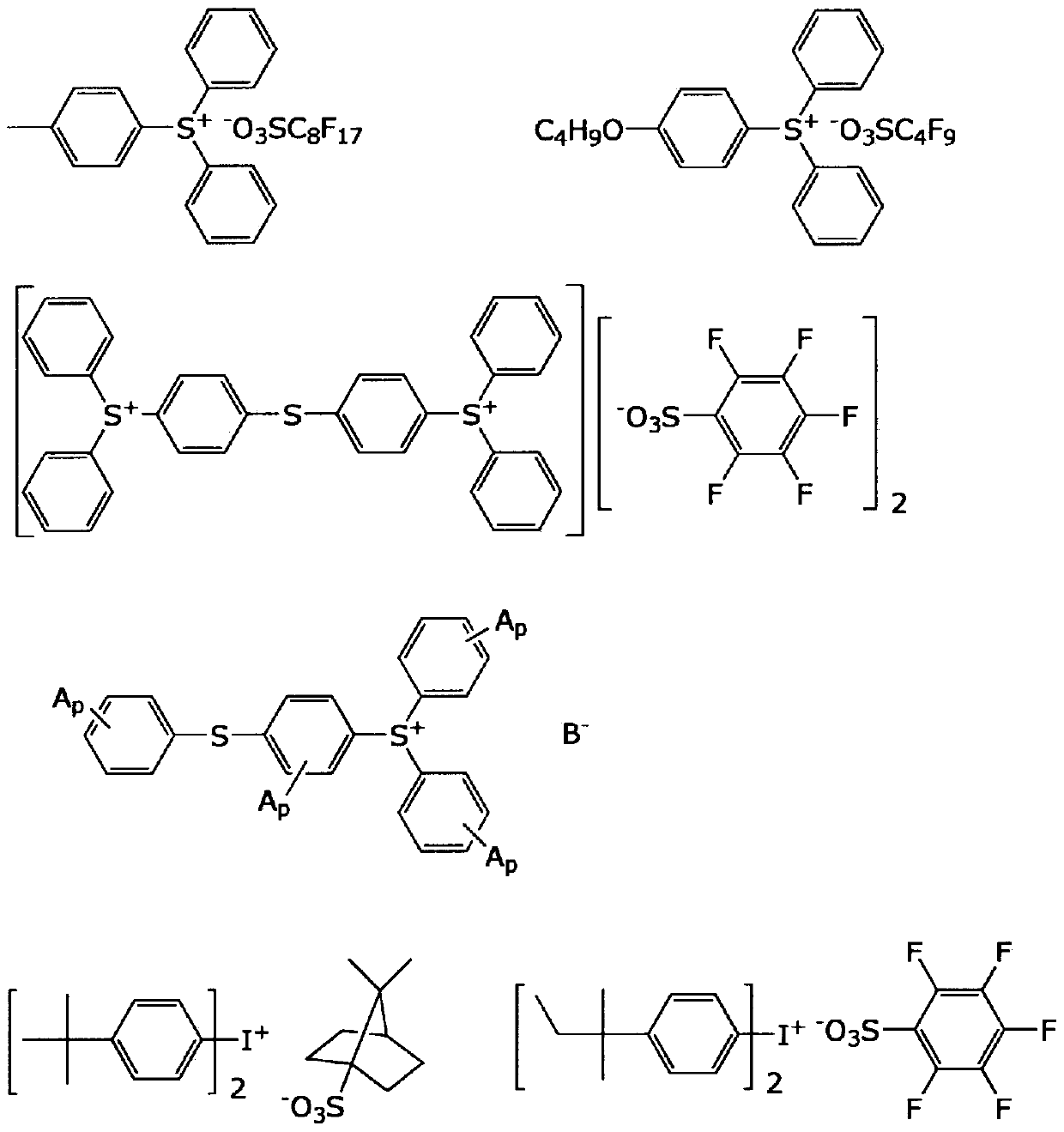
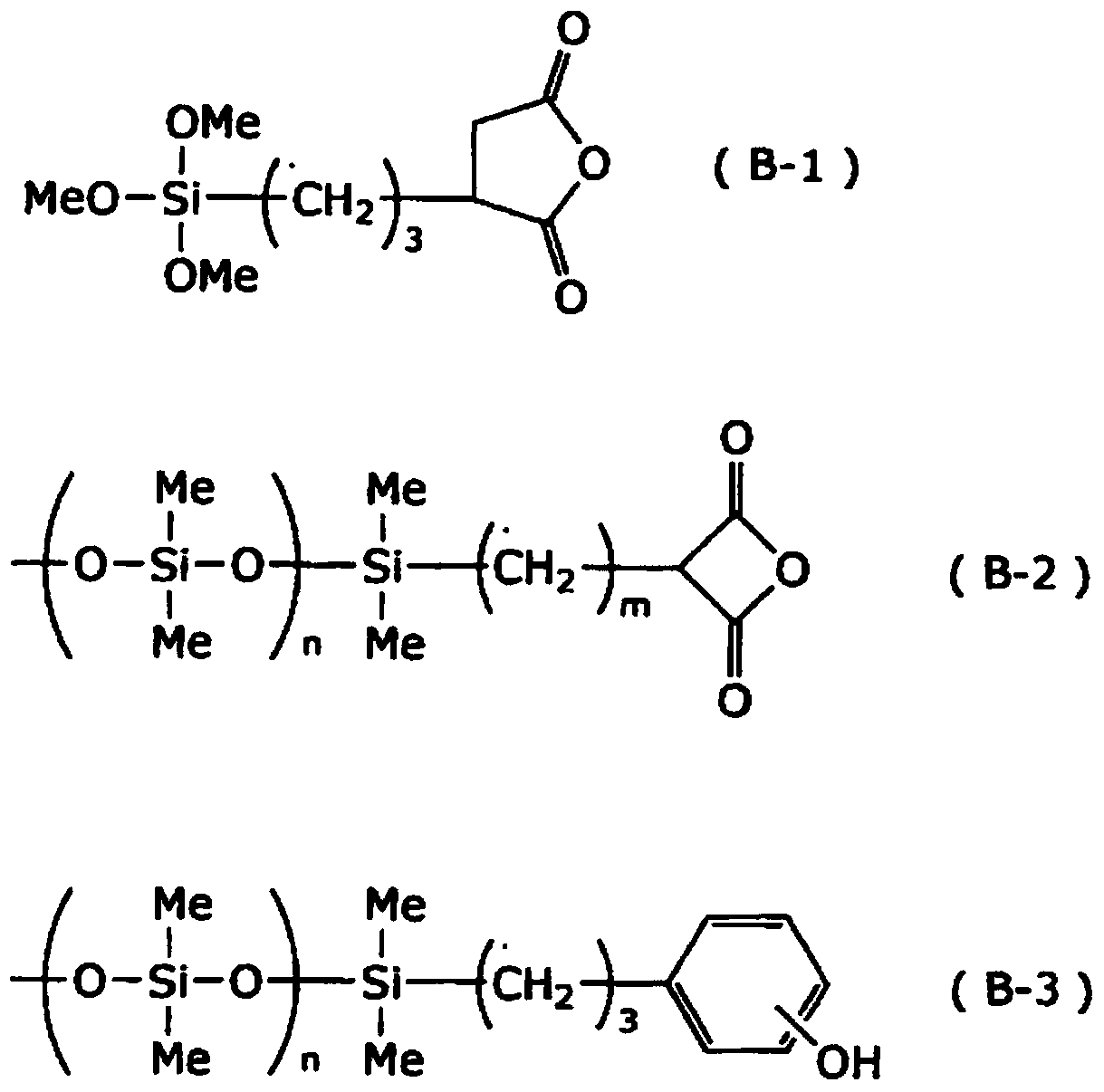
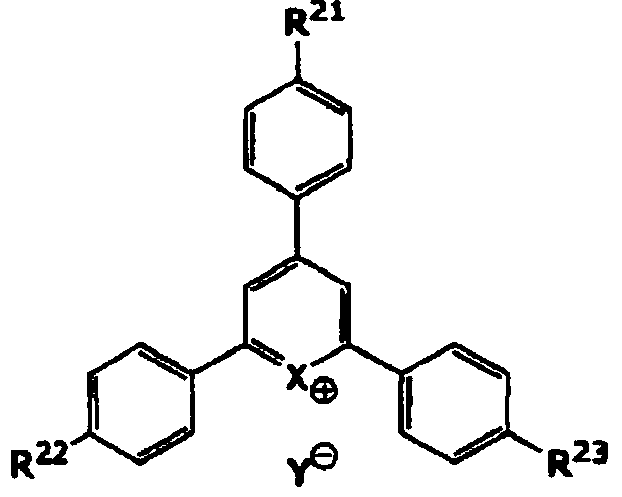
[0186] Polysiloxane S1, polyoxysilane S2, vinyl acrylic polymer A1-1 (weight average molecular weight 12,000, double bond equivalent 24g / eq, acid value 19mgKOH / g), carboxyl acrylic polymer A2- 1 (weight average molecular weight 7,500, acid value 120-130 mgKOH / g) was mixed at a ratio of 10:20:40:30 to obtain a polymer mixture. Regarding this polysiloxane mixture S1, the dissolution rate relative to 2.38% TMAH aqueous solution after prebaking is / sec, the weight-average molecular weight is 1,750, and for this polysiloxane mixture S2, the dissolution rate relative to the 5.00% TMAH aqueous solution after prebaking is / sec, and the weight average molecular weight is 2,700. To this polymer mixture, 25 parts by weight of tris(2-acryloyloxyethyl)isocyanurate (M1) and dipentaerythritol hexaacrylate (M2) were added as a (meth)acryloyl compound. 12 parts by weight, 6.0 parts by weight of Irgacure OXE-02 (PI) as a polymerization initiator, and 8 parts by weight of silicon compound SQ...
Embodiment 2~13、 comparative example 1~4
[0191] With respect to Example 1, it evaluated similarly to Example 1 by preparing the negative photosensitive composition which changed the component as shown in Table 1. The obtained results are shown in Table 1.
[0192] 【Table 1】
[0193]
[0194] In the table:
[0195] Acrylic polymer A1-2: Acrylic polymer having a vinyl group (weight average molecular weight 10,000, double bond equivalent 105 g / eq, acid value 20 mgKOH / g)
[0196] Acrylic polymer A2-2: an acrylic polymer having a carboxyl group (weight average molecular weight: 15,000, acid value: 120 to 130 mgKOH / g)
[0197] The evaluation criteria for each characteristic are as follows:
[0198] Residue after development
[0199] The film surface after development was observed and evaluated with the optical microscope.
[0200] A: No residue
[0201] B: There are some residues on the lower side of the pattern
[0202] C: Has a thin layer of residue
[0203] D: with thick layered residue
[0204] Residual...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Double bond equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com