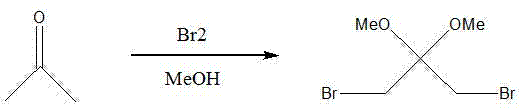Method for synthesizing 1,3-dibromo-2,2-dimethoxy propane
A technology of dimethoxypropane and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve problems such as large pollution and safety threats to staff, and achieve low cost of raw materials, easy operation, The effect of fewer process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 986 grams of acetone and 6 kilograms of methanol in a 10L reactor, and stir evenly. Slowly add 1.5 kg of bromine dropwise. After the reaction solution becomes transparent, add 3.8 kg of bromine dropwise. During the dropwise addition, the reaction solution is light reddish brown, and the temperature is not higher than 20°C. After the dropwise addition was completed, the mixture was stirred at room temperature for about 24 hours, and a large amount of off-white solid was precipitated. Centrifuge to remove solvent, 1.5 kg of methanol beating and washing twice to obtain white powdery crystals, after vacuum drying for 24 hours, 2.7 kg of product was obtained, with a molar yield of 66.68%, and a purity of 99% as determined by gas chromatography.
Embodiment 2
[0019] Pump 75.4 kg of methanol and 14.3 kg of acetone into the 200L reactor, and stir. Pump 79.0 kg of bromine into the header tank, slowly drop it into the reaction kettle, the temperature is not higher than 24°C, and continue stirring for 36 hours after the dropwise addition. The reaction solution was suction-filtered, and the filter cake was washed twice with 20 kg of methanol, and the obtained white crystal was the product, which was vacuum-dried to obtain 39 kg of the product, which was determined by gas chromatography with a purity of 98.9%.
Embodiment 3
[0021] Pump 950 kg of methanol and 180 kg of acetone into the 3000L reactor, and stir. Pump 1,000 kg of bromine into the header tank, slowly drop it into the reaction kettle, the temperature is not higher than 25°C, and continue stirring for 40 hours after the dropwise addition. The reaction solution was suction-filtered, and the filter cake was washed twice with 150 kilograms of methanol. The white crystals obtained were the product, and vacuum-dried to obtain 580 kilograms of the product. The purity of the product measured by gas chromatography was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com