Salts of epidermal growth factor receptor kinase inhibitor
A technology of hydrochloric acid and hydrobromide, which can be used in polymorphic form to treat various diseases and can solve problems such as dose-limiting toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0257] Preparation of compound 1
[0258] The synthesis of compound 1 is described in detail in Example 3 of the '061 application.
[0259]
[0260] step 1:
[0261] Pre-equipped with electromagnetic stirrer, thermal bag and CaCl 2 N-Boc-1,3-diaminobenzene (0.96 g) and n-butanol (9.00 mL) were charged into a 25 mL 3-neck round bottom flask with a protective tube. The reaction mixture was cooled to 0°C. At 0°C, 2,4-dichloro-5-trifluoromethylpyrimidine (1.0 g) was added dropwise to the above reaction mixture. At 0°C, diisopropylethylamine (DIPEA) (0.96 mL) was added dropwise to the above reaction mixture and the reaction mixture was stirred at 0°C to 5°C for 1 hour. Finally, the reaction mixture was allowed to warm to room temperature. The reaction mixture was stirred for another 4 hours at room temperature. Using hexane: ethyl acetate (7:3), the completion of the reaction was monitored by TLC. The precipitated solid was filtered off and washed with 1-butanol (2 mL). The solid ...
example 1
[0288] Primary salt screening
[0289] Based on the general preparation of compound 2, the results of the primary salt screening are shown in Table 1. Table 1 indicates the relative ions, solvents and resulting solid forms.
[0290] Table 1. Results of primary salt screening
[0291]
[0292] S1 = salt, polymorph form 1
[0293] S2 = salt, polymorphic form 2
[0294] S3 = salt, polymorphic form 3
[0295] S4 = salt, polymorphic form 4
[0296] SP = salt, partially crystalline
[0297] FA = free acid
[0298] FC = free compound 1
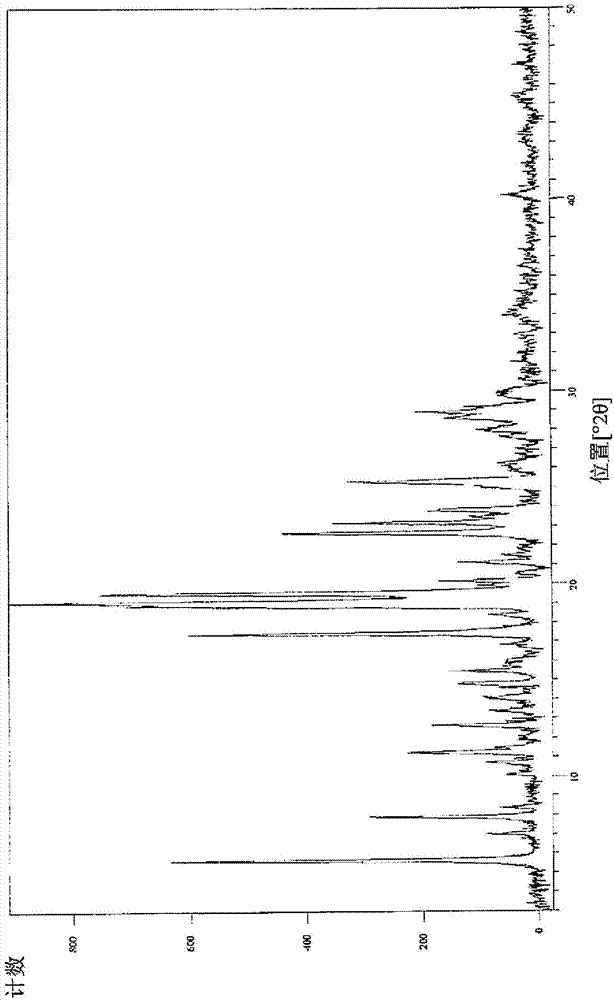
[0299] XR=Different XRPD patterns, but only a few peaks in the diffraction pattern (may indicate degradation)
[0300] AM = amorphous
[0301] GM = solid that quickly turns into a jelly after separation
example 2
[0303] Primary salt screening
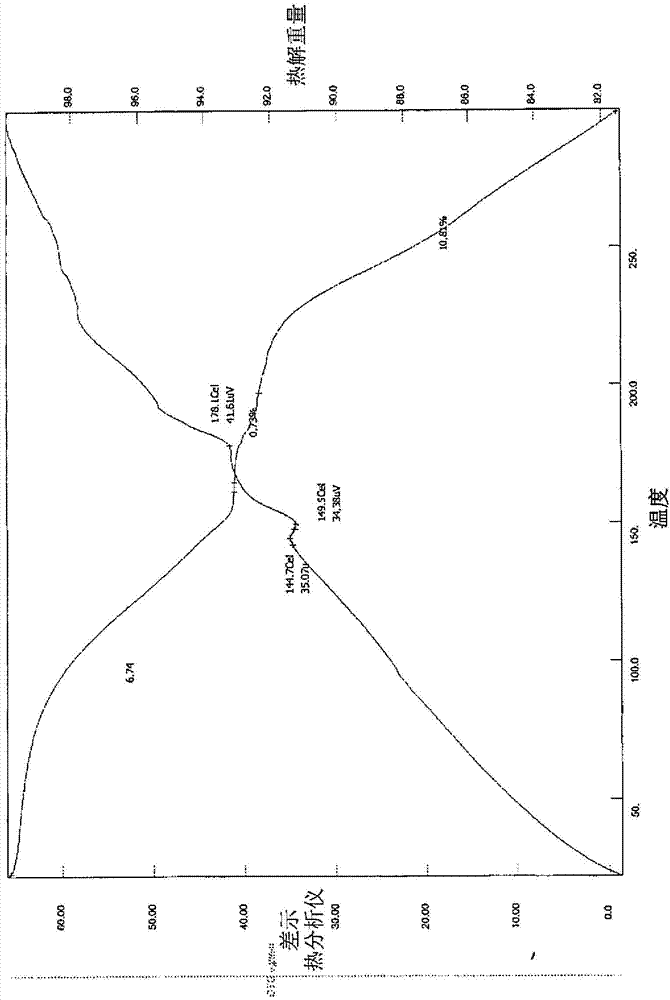
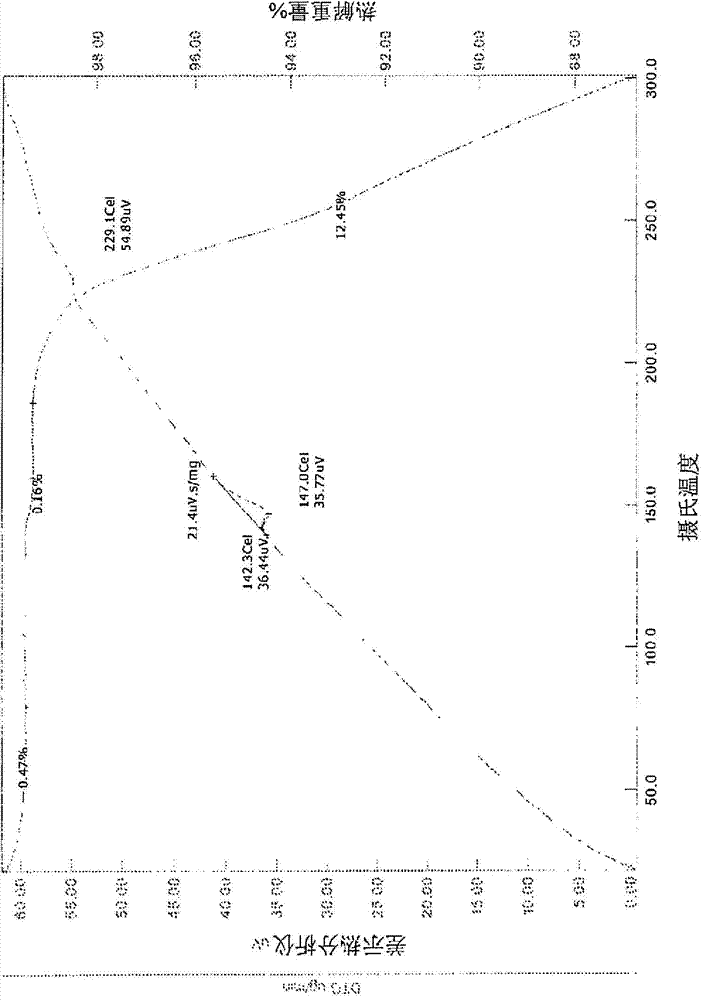
[0304] For the potential salt obtained during the primary salt screening in Example 1, the sample was set at 40°C / 75% RH (open vial) and 80°C (open vial) for a 1-week stability study. After the stability study, TGA is performed on samples containing sufficient substances. Still in aqueous medium (pH <2) Test the solubility of the sample. The results of the stability and solubility studies are indicated in Table 2.
[0305] Table 2. Stability and solubility results of potential salts obtained in primary salt screening
[0306]
[0307]
[0308]
[0309] Based on these results, bis-benzenesulfonate was selected and acetone was used as a solvent to increase it proportionally. In addition, the hydrobromide salt is selected, and acetonitrile: water (90:10) is used as a solvent to increase it proportionally. The mono-maleate and bis-hydrochloride salts were also selected for scale-up experiments to assess whether these salts were solvated / hydrated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com