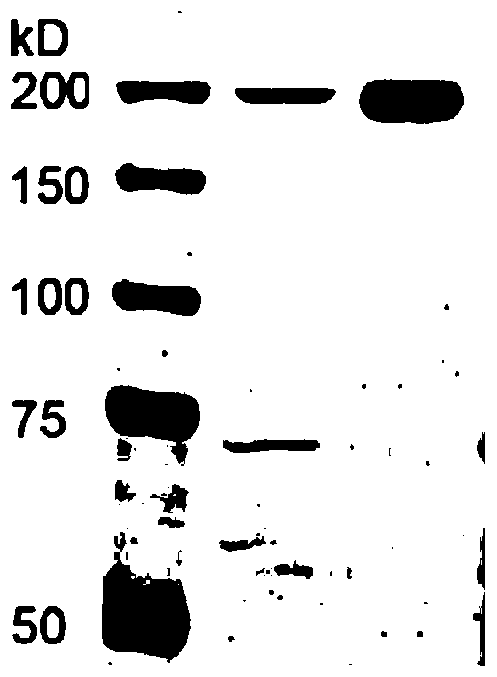A kind of fully human anti-ctla-4 monoclonal antibody, preparation method and application
A CTLA-4, monoclonal antibody technology, applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-tumor drugs, etc., to achieve extensive and efficient anti-infection and treatment of autoimmune diseases Expression and inhibition of tumor cell growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
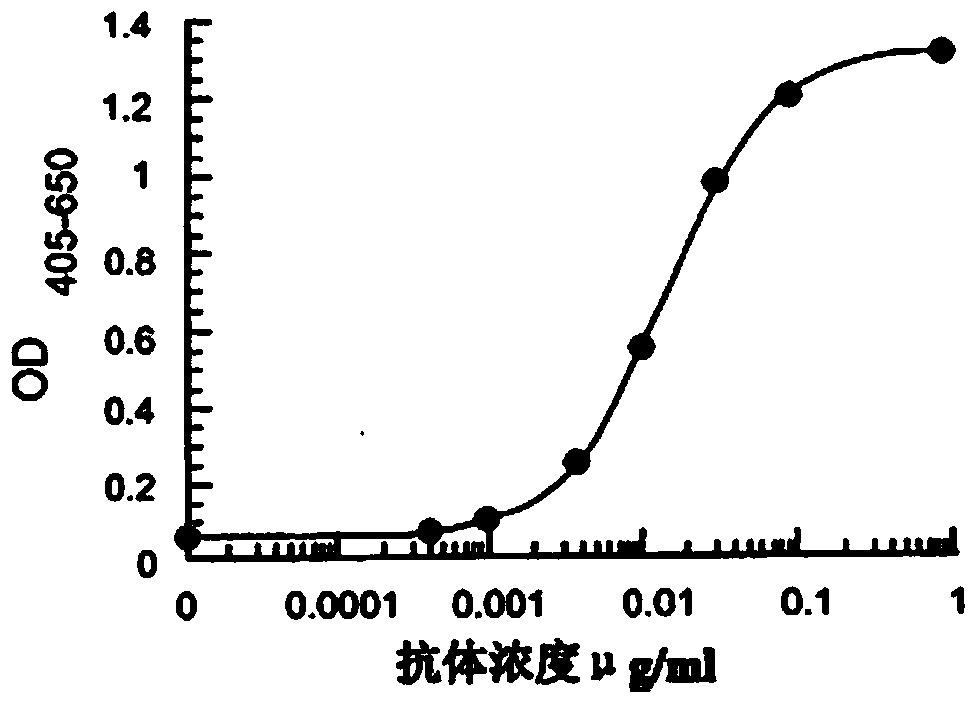
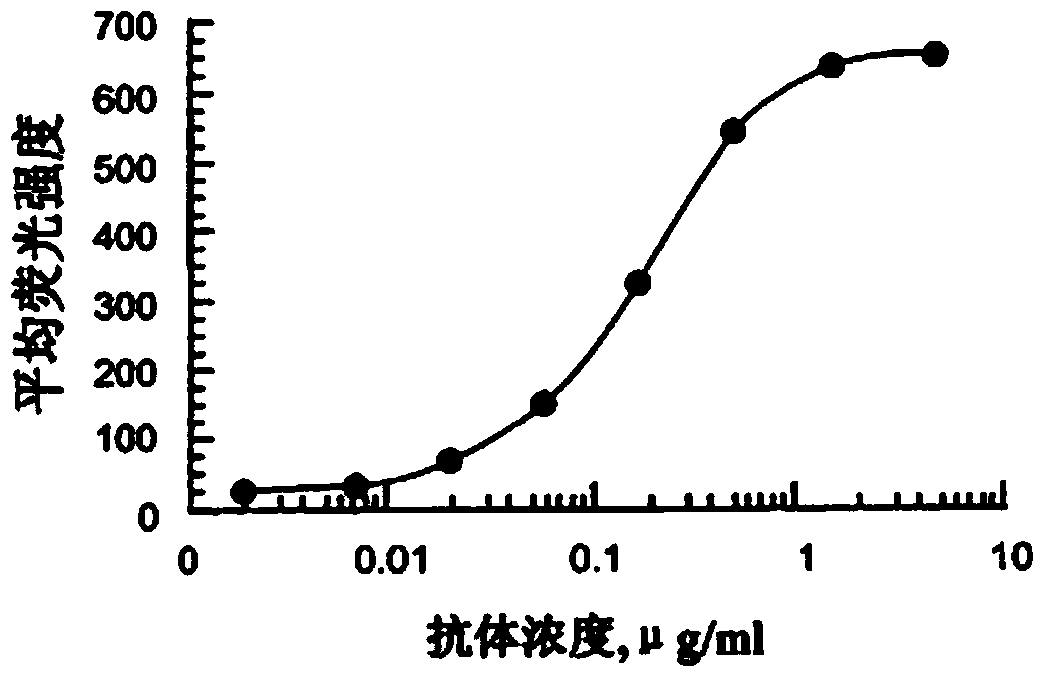
Image
Examples
Embodiment 1
[0033] (1) Preparation of cDNA
[0034] Collect 10 ml of peripheral blood from 50 people with liver cancer, kidney cancer, melanoma, and healthy people, anticoagulate with sodium heparin, separate mononuclear cells (PBMC) by density gradient centrifugation, and sensitize with CTLA-4 antigen in vitro. After PBMC were sensitized in vitro, press 4×10 6 Add 1ml of EBV culture supernatant to PBMC, incubate at 37°C for 3h, discard the supernatant, add RPMI1640 complete medium containing 100ml / LFCS, culture for 2 weeks, replace with RPMI1640 complete medium containing 200ml / L NBS. The culture supernatant of transformed cells was screened by indirect ELISA method, CTLA-4 was used as the tumor cell antigen solid phase plate, the collected B cell culture supernatant was used as the primary antibody, and HRP-labeled goat anti-human IgG / M (HRP-GAH-IgG / M) as the secondary antibody. Cells from positive wells were expanded to 10 6 ~10 7 cells.
[0035] (2) PCR amplification and connect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com