Microencapsulated daily use essence and preparation method thereof
A technology for microcapsules and daily use, which is applied in the direction of microcapsule preparation, preparation of microspheres, essential oils/fragrances, etc. It can solve the problems of poor stability of microcapsule daily essences, and achieve the purpose of prolonging the fragrance retention time, ensuring safety, and system stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
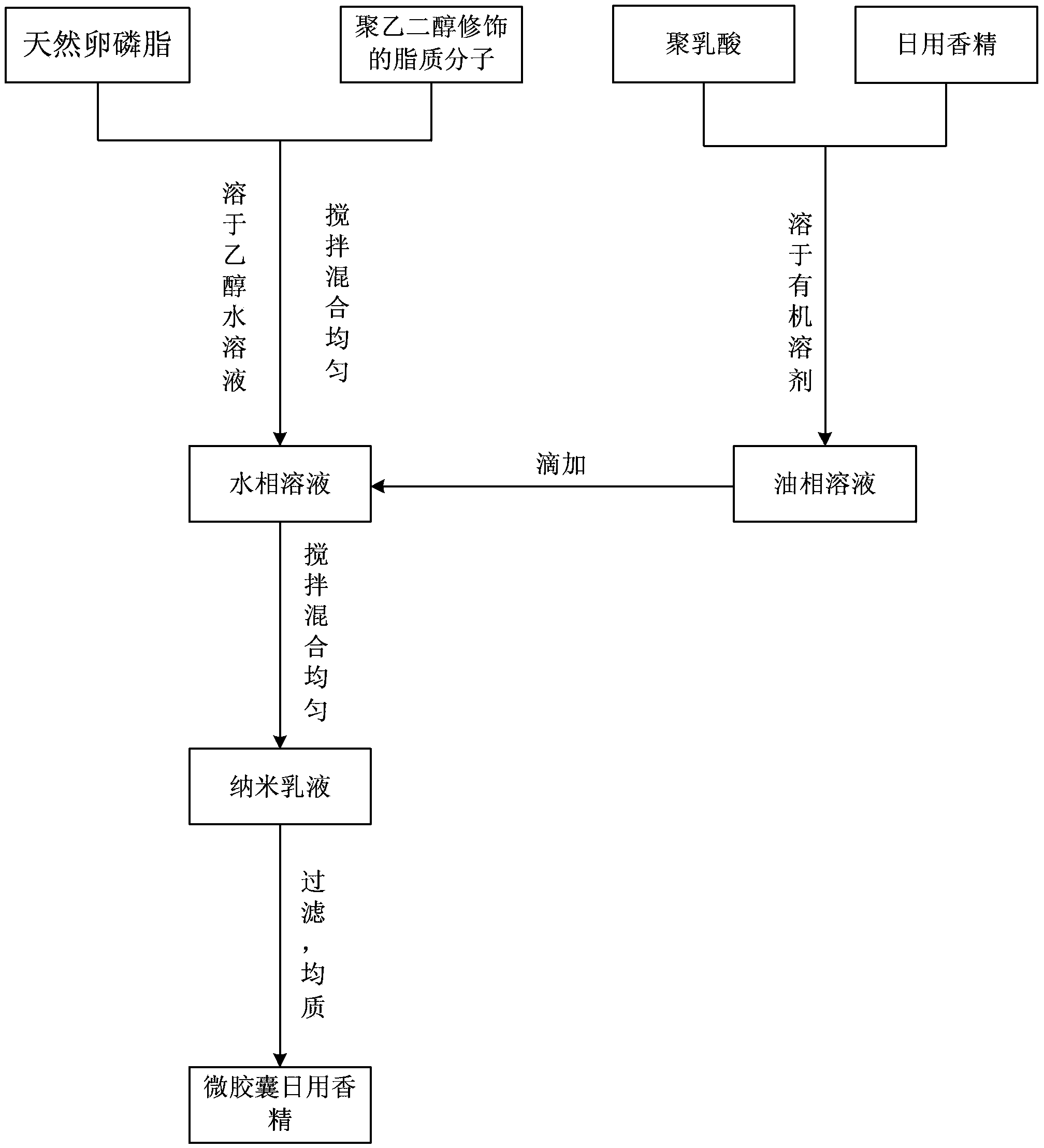
[0065] A kind of microcapsule daily essence, its preparation method comprises the following steps:
[0066] (1) Dissolve 3.19mg of soybean lecithin and 1.31mg of polyethylene glycol-distearoylphosphatidylethanolamine (PEG-DSPE) in 30mL of ethanol solution (volume fraction of ethanol is 4%), and heat in a water bath , stirred evenly by magnetic force until completely dissolved to form a transparent solution, and an aqueous phase solution containing lipid components was obtained;
[0067] In the aqueous phase solution, the molar ratio of soybean lecithin and PEG-DSPE is 9:1; the concentration of lipid components (soybean lecithin and PEG-DSPE) is 0.15 mg / mL;
[0068] (2) Dissolve 25.5mg of polylactic acid (PLGA) and 15mg of daily oil-soluble rose essence into 8.1mL of tetrahydrofuran solution, and magnetically stir until completely dissolved to obtain an oil phase solution;
[0069] The mass ratio of the PLGA to the lipid component is 8.5:1.5; the mass ratio of the daily essenc...
Embodiment 2
[0074] A kind of microcapsule daily essence, its preparation method comprises the following steps:
[0075] (1) Dissolve 2.73mg of soybean lecithin and 1.78mg of polyethylene glycol-distearoylphosphatidylethanolamine (PEG-DSPE) in 30mL of ethanol solution (volume fraction of ethanol is 4%), and heat in a water bath , stirred evenly by magnetic force until completely dissolved to form a transparent solution, and an aqueous phase solution containing lipid components was obtained;
[0076] In the aqueous phase solution, the molar ratio of soybean lecithin and PEG-DSPE is 8.5:1.5; the concentration of lipid components (soybean lecithin and PEG-DSPE) is 0.15mg / mL;
[0077](2) Dissolve 25.5mg of polylactic acid (PLGA) and 15mg of daily oil-soluble rose essence into 8.1mL of tetrahydrofuran solution, and magnetically stir until completely dissolved to obtain an oil phase solution;
[0078] The mass ratio of the PLGA to the lipid component is 8.5:1.5; the mass ratio of the daily esse...
Embodiment 3
[0083] A kind of microcapsule daily essence, its preparation method comprises the following steps:
[0084] (1) Dissolve 1.3mg of soybean lecithin and 3.2mg of polyethylene glycol-distearoylphosphatidylethanolamine (PEG-DSPE) in 30mL of ethanol solution (volume fraction of ethanol is 4%), and heat in a water bath , stirred evenly by magnetic force until completely dissolved to form a transparent solution, and an aqueous phase solution containing lipid components was obtained;
[0085] In the aqueous phase solution, the molar ratio of soybean lecithin and PEG-DSPE is 6:4; the concentration of lipid components (soybean lecithin and PEG-DSPE) is 0.15 mg / mL;
[0086] (2) Dissolve 25.5mg of polylactic acid (PLGA) and 15mg of daily oil-soluble rose essence into 8.1mL of tetrahydrofuran solution, and magnetically stir until completely dissolved to obtain an oil phase solution;
[0087] The mass ratio of the PLGA to the lipid component is 8.5:1.5; the mass ratio of the daily essence ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com