A method and system for removing impurities from lepidolite leachate
A leachate and lepidolite technology, applied in the field of metallurgy, can solve the problems of reducing energy consumption, unwillingness to change the status quo, high loss rate of lithium, rubidium, and cesium, and achieve the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
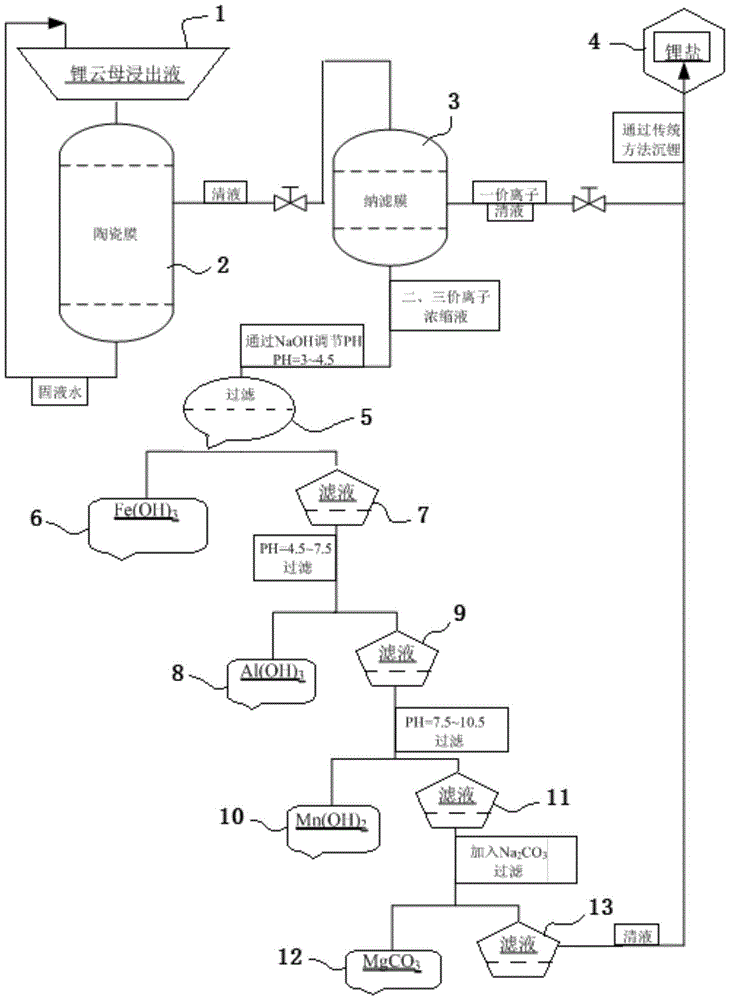
[0038] Embodiment 1: get a part of 48L above-mentioned solution, adjust pH value to be 2-2.5 (if greater than 2.5 because of pH value, there will be Fe(OH) 3 Precipitate), in the nanofiltration membrane that pressure is 25MPa, carry out the separation of monovalent ion and multivalent ion, obtain filtrate 42L (if continue to separate, then filtration speed is too slow, energy consumption increases), lithium permeability is 78.5%. After step-by-step precipitation in the stock solution bucket, the lithium in the stock solution lost 3.1%. The total yield was 96.9% and the lithium loss rate was 3.1% during the impurity removal process.
Embodiment 2
[0039] Embodiment 2: Get a portion of the above-mentioned solution of 48L, and adjust the pH value to be 2-2.5 (if the pH value is greater than 2.5, there will be Fe(OH) 3 Precipitate), in the nanofiltration membrane that pressure is 30MPa, carry out the separation of monovalent ion and polyvalent ion, obtain filtrate 42L (if continue to separate, then filtration speed is too slow, energy consumption increases), lithium permeability is 80.3%. After step-by-step precipitation in the stock solution bucket, 2.9% of lithium was lost in the stock solution. The total yield was 97.1% and the lithium loss rate was 2.9% during the impurity removal process.
Embodiment 3
[0040] Embodiment 3: get a part of 48L above-mentioned solution, adjust pH value to be 2-2.5 (if greater than 2.5 because of pH value, there will be Fe(OH) 3Precipitation), the separation of monovalent ions and polyvalent ions was carried out in a nanofiltration membrane with a pressure of 35MPa to obtain 42L of filtrate, and the lithium permeability was 82.6%. After step-by-step precipitation in the stock solution bucket, 2.6% of lithium was lost in the stock solution. The total yield was 97.4% and the lithium loss rate was 2.6% during the impurity removal process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com