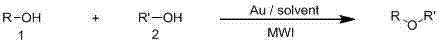
Synthetic method of asymmetrical ether by gold-catalysis
A synthetic method and asymmetric technology, applied in the field of synthesis of gold-catalyzed asymmetric ethers, achieving good yield, high product purity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Phenethyl alcohol (0.5 mmol, 61.2 mg) and benzyl alcohol (1.5 mmol, 162.1 mg) were added to a 10 mL microwave tube, and Ph 3 PAuCl (0.00025mmol, 0.2mg), the reaction mixture was heated to 150° C. with a microwave reactor, stirred for 90 minutes, and after completion of the reaction, the target product I was isolated by flash column chromatography with a yield of 96%.
[0031] The reaction formula of benzyl alcohol and phenethyl alcohol is:
[0032]
[0033] The spectral data of product I are: ESI-MS (m / z): 213 [M+H] + ; 1 H-NMR (600MHz, CDCl 3 )δ7.41-7.44(m,5H),7.34-7.38(m,3H),7.26-7.30(m,2H),4.58(s,2H),3.76(t,J=7.2Hz,2H),3.00 (t,J=7.2Hz,2H).
Embodiment 2
[0035] Using cyclohexanol instead of phenethyl alcohol, the other is the same as in Example 1, to obtain the target compound II with a yield of 76%. The reaction formula of benzyl alcohol and cyclohexanol is:
[0036]
[0037] The spectral data of product II are: ESI-MS (m / z): 191 [M+H] + ; 1 H-NMR (600MHz, CDCl 3)δ7.32–7.38(m,4H),7.25–7.28(m,1H),4.56(s,2H),3.33–3.40(m,1H),1.94–1.96(m,2H),1.76–1.78( m,2H),1.51–1.57(m,1H),1.34–1.40(m,2H),1.26–1.28(m,2H),0.83–0.92(m,1H).
Embodiment 3
[0039] Using tert-butyldimethylsilanol instead of phenethyl alcohol, the others were the same as in Example 1 to obtain the target compound III with a yield of 67%. The reaction formula of benzyl alcohol and tert-butyldimethylsilanol is:
[0040]
[0041] The spectral data of product III is: ESI-MS (m / z): 237[M+H] + ; 1 H-NMR (600MHz, CDCl 3 )δ7.36(d,J=4.5Hz,4H),7.27–7.29(m,1H),4.79(s,2H),0.99(s,9H),0.14(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com