4aβ1‑15 derived monoclonal antibody and its application
A monoclonal antibody, antibody technology, applied in the field of immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0051] Example 1 Synthesis of 4Aβ1-15 peptide
[0052] For the preparation method of 4Aβ1-15 peptide, please refer to the patent No. "ZL 201010163165.2" and the invention patent named "High-efficiency secretion expression and purification method of recombinant 4Aβ15 in Pichia pastoris", wherein 4Aβ15 is the 4Aβ1-15 of the present invention . The 4Aβ1-15 polypeptide antigen used in the present invention is provided by Livzon Pharmaceutical Company, and the protein purity is >95%.
[0053] In addition, the full-length 4Aβ1-15 peptide can also be artificially synthesized according to the sequence of 4Aβ1-15, wherein:
[0054] The amino acid sequence of Aβ1-15 peptide is: DAEFRHDSGYEVHHQ;
[0055] The amino acid sequence of the 4Aβ1-15 peptide is: 4 repeating sequences of DAEFRHDSGYEVHHQ, connected by the peptide segment GGPPG in the middle, with a total of 75 amino acids.
Embodiment 2
[0056] Example 2 Production of hybridoma and preparation of monoclonal antibody
[0057] animal immunity
[0058] 6-week-old Balb / c mice were immunized with purified 4Aβ1-15 antigen (protein purity>95%). The way of immunization is subcutaneous or intraperitoneal injection. For the first immunization, the antigen was emulsified with Freund's complete adjuvant (FCA; Sigma), and the immunization dose was 50 μg per mouse. After an interval of two weeks, the antigen was emulsified with Freund's incomplete adjuvant (FIA; Sigma) for the second immunization with a dose of 50 μg per mouse. Two weeks after the second immunization, the antigen was emulsified with Freund's incomplete adjuvant (FIA; Sigma) for the third immunization, and the immunization dose was 50 μg per mouse. Two weeks after the third immunization, a booster immunization was carried out, and the immunization dose was 100 μg / bird. Among them, the polypeptide antigen is dissolved in PBS at 1 mg / ml, and the adjuvant...
Embodiment 3
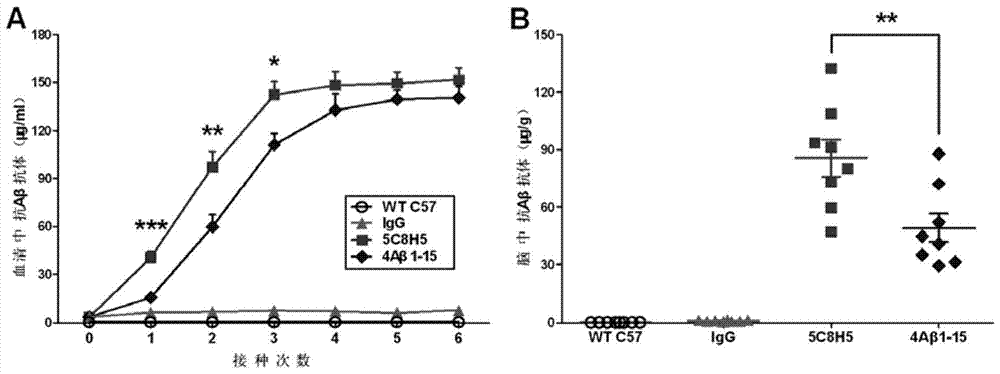
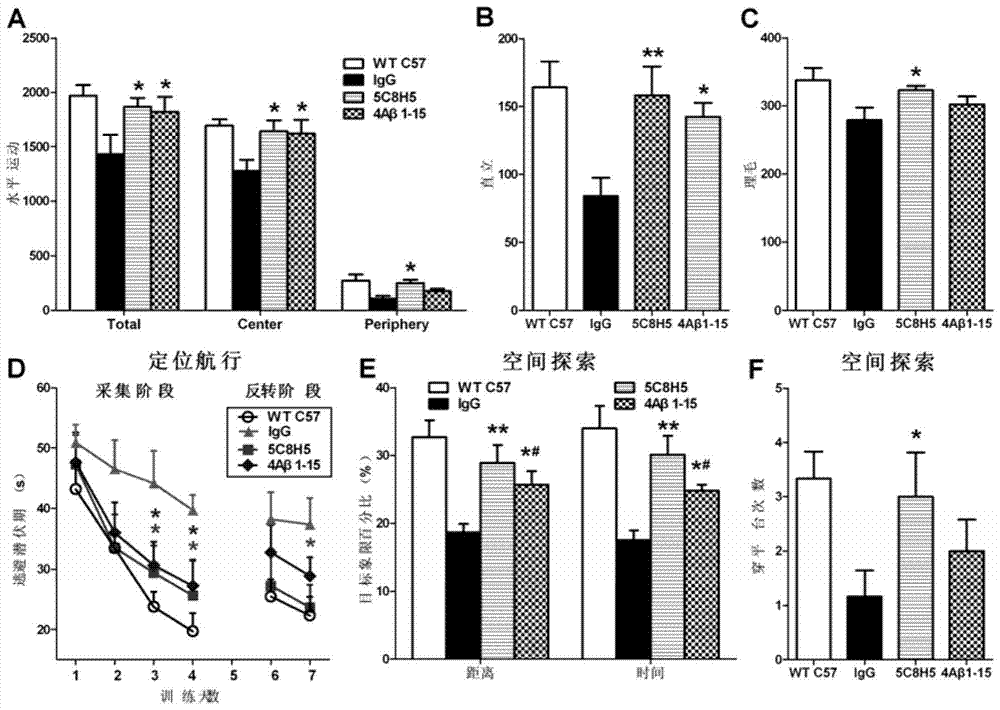
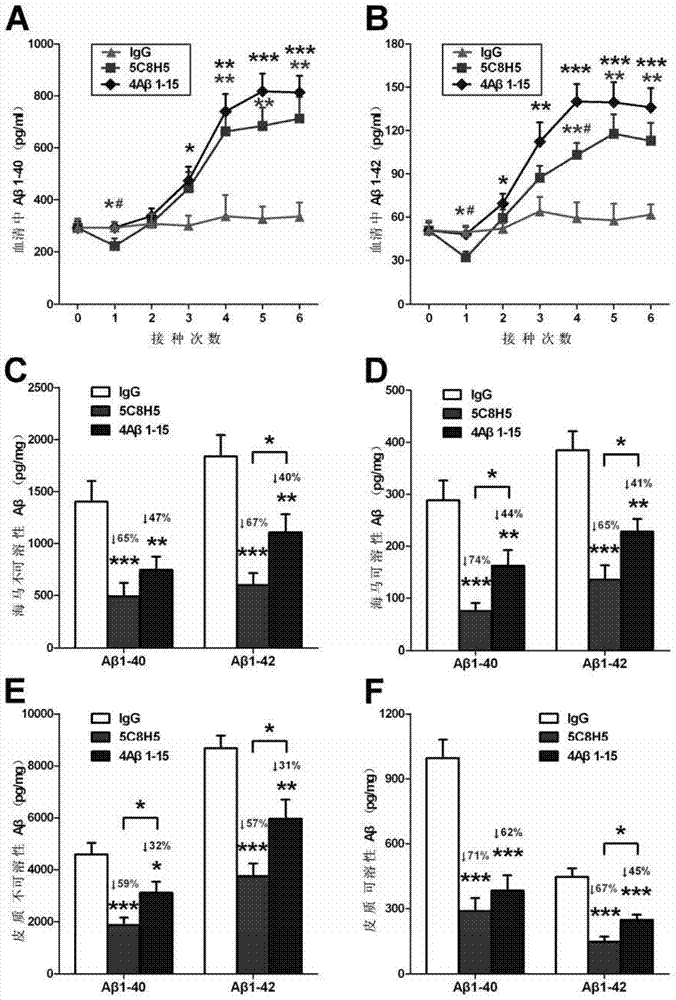
[0089] Example 3 Immunology, Pathology and Behavioral Observation Tests of AD Transgenic Mice Inoculated with Monoclonal Antibody
[0090] 1. Materials and methods
[0091] 1.1 Grouping and immunization procedures
[0092] The mice were divided into 4 groups (8 in each group), three of which were AD transgenic model mice APP / PS1 provided by the Model Animal Research Center of Nanjing University, and the other group were wild-type C57Bl / 6J mice without treatment. Among the three groups of transgenic mice, one group was inoculated with the monoclonal antibody 5C8H5 (hereinafter referred to as "5C8H5") as the experimental group. Since 5C8H5 was identified as an IgG phenotype, we chose mouse IgG, an irrelevant isotype control antibody, as a negative control, that is, the second group was vaccinated with mouse IgG. At the same time, we established a group of mice inoculated with 4Aβ1-15 antigen to compare whether 4Aβ1-15 and 5C8H5 under the same immunization procedure, that is,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com