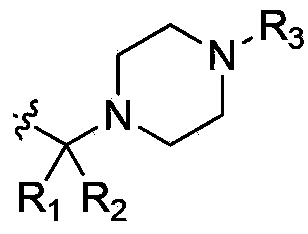
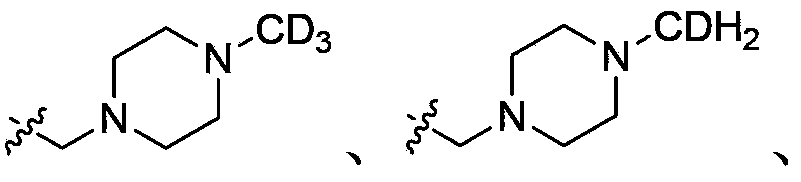
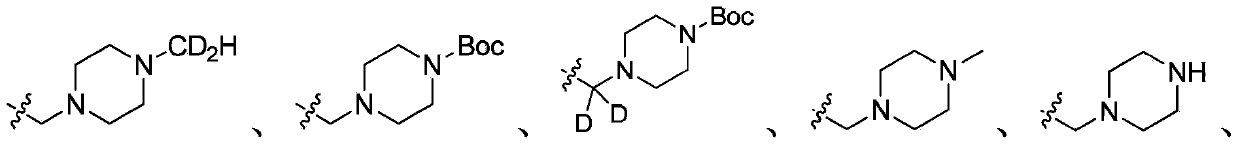
Deuterated acetylenic derivative, pharmaceutical composition and application thereof
A technology of deuterated acetylene and derivatives, which can be used in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., and can solve the problems of affecting clinical medication, poor pharmacokinetic properties, and large toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0270] Example 1: 4-Difluoromethyl-3-imidazol[1,2-b]pyridazin-3-ylethynyl-N-[4-(4-trideuteromethyl-piperazin-1-yl Methyl)-3-trifluoromethyl-phenyl]-benzamide
[0271]
[0272] The first step: methyl 3-iodo-4-bromomethylbenzoate
[0273] Dissolve methyl 3-iodo-4-methylbenzoate (200 mg, 0.7 mmol) in carbon tetrachloride (5 mL), add N-bromosuccinimide (180 mg, 1.0 mmol) and benzene peroxide in sequence Formyl (17.5mg, 0.07mmol), stirred at 100°C for 24h. After the reaction, the solvent was evaporated, the residue was adjusted to pH 9 with saturated sodium bicarbonate solution, extracted twice with ethyl acetate, the organic layers were combined, washed with water, washed with saturated brine, dried, and concentrated under reduced pressure to obtain a crude reddish-brown oil. It was directly used in the next reaction without further purification.
[0274] The second step: methyl 3-iodo-4-hydroxymethylbenzoate
[0275] The above crude 3-iodo-4-bromomethylbenzoate (300mg) was...
Embodiment 2~ Embodiment 19
[0291] Using different aryl halides and aryl acetylenes as raw materials, the compound of Example 2 to Compound 19 of Example were synthesized by general method 1, and the specific chemical structure identification data are shown in Table 1.
[0292] Table 1. Chemical Structure Identification Data of Example Compound 2~Example Compound 19
[0293]
[0294]
[0295]
[0296]
[0297]
Embodiment 20~ Embodiment 25
[0299] Using Example Compounds 5, 6, 10, 11, 12, and 19 in Table 1 as raw materials, Example Compound 20 to Example Compound 25 were synthesized by General Method 2. The specific chemical structure identification data are shown in Table 2.
[0300] Table 2. The chemical structure identification data of Example Compound 20~Example Compound 25
[0301]
[0302]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com