Method for extracting lithium from salt lake brine
A salt lake brine and extraction technology, applied in the chemical industry and extraction chemistry field, can solve the problems of solvent loss of extraction agent, strong corrosion of extraction equipment, and restrictions on large-scale industrial application, etc., to reduce concentration, improve strong corrosion, and simple operation reliable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
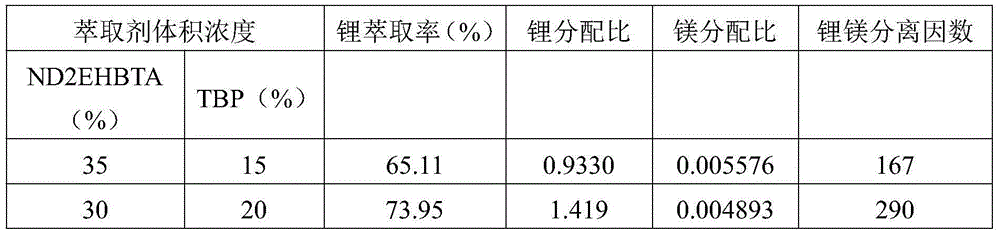
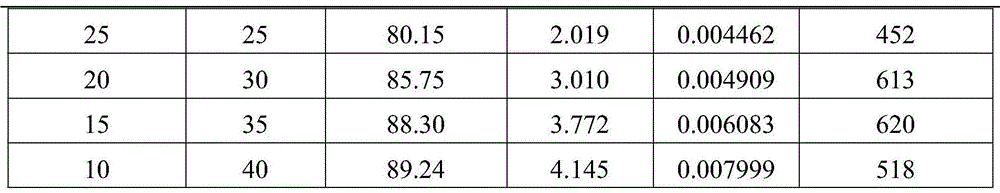
Image
Examples
Embodiment 1
[0017] The method for extracting lithium in this salt lake brine provided by the present embodiment includes:
[0018] 1) Prepare the organic phase for extraction: take 300mL of tributyl phosphate and 700mL of N,N-bis(2-ethylhexyl)-3-butanoneacetamide to form a composite extractant, take 1000mL of sulfonated kerosene as a diluent and add to the compound in the extractant;
[0019] 2) Extract the water phase: take the salt lake brine of saturated magnesium chloride solution, which is LiCl-MgCl 2 -H 2 O system;
[0020] 3) Add HCl, FeCl to the extracted aqueous phase 3 ·6H 2 O, wherein, the ratio of the amount of substances controlling iron and lithium is 1.3:1, at this moment, the lithium concentration is 1.9604g / L, and the acid concentration is 0.05mol / L;
[0021] 4) Mix the extracted aqueous phase obtained in step 3) with the extracted organic phase obtained in step 1) at a volume ratio of 1:2, fully mix and shake for 6 minutes, then stand still and separate the liquid p...
Embodiment 2
[0023] The method for extracting lithium in this salt lake brine provided by the present embodiment includes:
[0024] 1) Prepare the organic phase for extraction: take 400mL of tributyl phosphate and 600mL of N,N-bis(2-ethylhexyl)-3-butanoneacetamide and mix them into a composite extractant, take 1000mL of sulfonated kerosene as diluent and add to the compound in the extractant;
[0025] 2) Extract the water phase: take the salt lake brine of saturated magnesium chloride solution, which is LiCl-MgCl 2 -H 2 O system;
[0026] 3) Add HCl, FeCl to the extracted aqueous phase 3 ·6H 2 O, wherein, the ratio of the amount of substances controlling iron and lithium is 1.3:1, at this moment, the lithium concentration is 1.9604g / L, and the acid concentration is 0.05mol / L;
[0027] 4) Mix the extracted aqueous phase obtained in step 3) with the extracted organic phase obtained in step 1) at a volume ratio of 1:2, fully mix and shake for 6 minutes, then stand still and separate the ...
Embodiment 3
[0029] The method for extracting lithium in this salt lake brine provided by the present embodiment includes:
[0030] 1) Prepare the organic phase for extraction: take 500mL of tributyl phosphate and 500mL of N,N-bis(2-ethylhexyl)-3-butanoneacetamide and mix them into a composite extractant, take 1000mL of sulfonated kerosene as a diluent and add to the compound in the extractant;
[0031] 2) Extract the water phase: take the salt lake brine of saturated magnesium chloride solution, which is LiCl-MgCl 2 -H 2 O system;
[0032] 3) Add HCl, FeCl to the extracted aqueous phase 3 ·6H 2 O, wherein, the ratio of the amount of substances controlling iron and lithium is 1.3:1, at this moment, the lithium concentration is 1.9604g / L, and the acid concentration is 0.05mol / L;
[0033] 4) Mix the extracted aqueous phase obtained in step 3) with the extracted organic phase obtained in step 1) at a volume ratio of 1:2, fully mix and shake for 6 minutes, then stand still and separate th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com