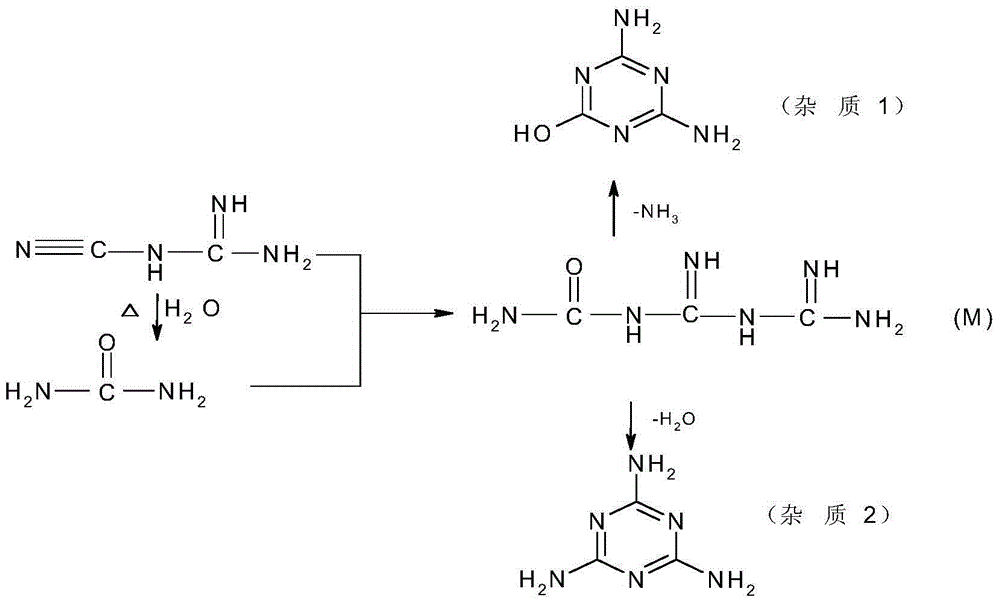
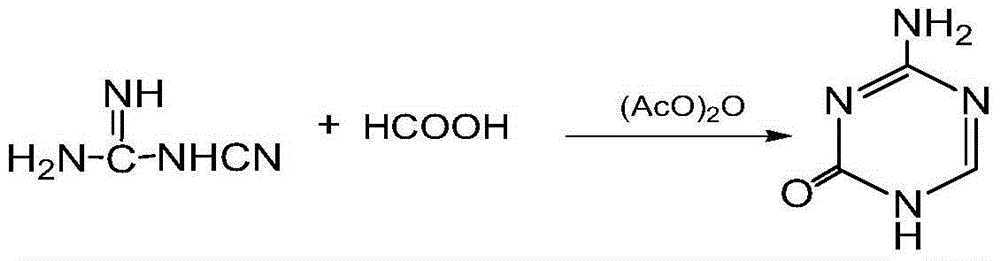Preparation method for 5-azacytosine
A technology of azacytosine and dicyandiamide, which is applied in the field of preparation of 5-azacytosine, can solve problems such as potential safety hazards, unsuitable application, and rising cost of final product raw materials, and achieve good process safety and no process wastewater Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Put 140 kg of formic acid into the reaction kettle, start stirring, put in 25 kg of dicyandiamide, the reaction temperature will rise gradually by itself, control the temperature below 60°C, and remove the remaining 75 kg of dicyandiamide in batches after the reaction temperature no longer rises. Add to the reaction kettle within 4-5 hours, control the temperature at 50-60°C and stir for 4 hours; then stop the temperature control measures, the reaction temperature will rise slowly by itself, and the temperature will rise to 100-110°C for 1 hour.
[0027] Add 120 kg of acetic anhydride dropwise to the reaction kettle, keep the temperature at 100-110°C during the dropwise addition, and stir at this temperature for 45 minutes after the dropwise addition; add 100 kg of acetic anhydride dropwise again, after the dropwise completion, raise the temperature to reflux, The reaction was maintained at reflux for 2 hours.
[0028] After the reflux is completed, stir and cool down t...
Embodiment 2
[0030] Put 140 kg of formic acid into the reaction kettle, start stirring, put in 25 kg of dicyandiamide, the reaction temperature will rise gradually by itself, control the temperature below 60°C, and remove the remaining 75 kg of dicyandiamide in batches after the reaction temperature no longer rises. Add to the reaction kettle within 4-5 hours, control the temperature at 50-60°C and stir for 4 hours; then stop the temperature control measures, the reaction temperature will rise slowly by itself, and the temperature will rise to 110-120°C for 1.5 hours.
[0031] Add 120 kg of acetic anhydride dropwise to the reaction kettle, keep the temperature at 100-110°C during the dropwise addition, and stir at this temperature for 45 minutes after the dropwise addition; add 100 kg of acetic anhydride dropwise again, after the dropwise completion, raise the temperature to reflux, The reaction was maintained at reflux for 2 hours.
[0032] After the reflux is completed, stir and cool dow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com