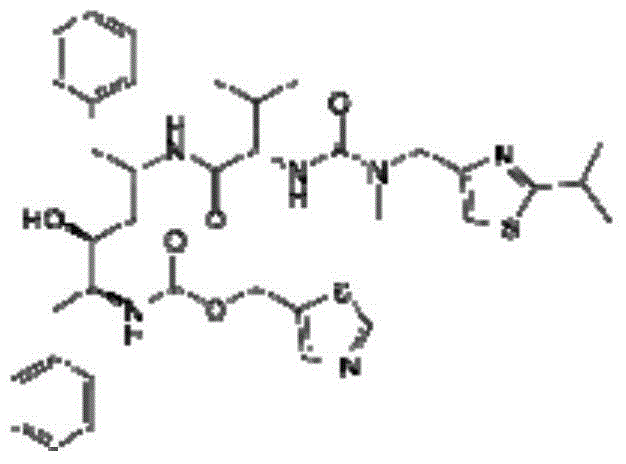
Antiretroviral composition
A technology of anti-retrovirus and composition, which can be used in anti-viral agents, drug delivery, microcapsules, etc., and can solve expensive problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] method:
[0153] (1) Prepare a dry mixture of lopinavir, ritonavir and colloidal silicon dioxide.
[0154] (2) Add sorbitan monolaurate to the copovidone in a suitable granulator to form a polymer premix separately.
[0155] (3) Mix the dry mixture obtained in step (1) and step (2) in a suitable granulator and melt extrude (hot).
[0156] (4) Colloidal silicon dioxide was mixed with dry granules and lubricated by using sodium stearyl fumarate.
[0157] (5) Compress the lubricated granules into microtablets.
[0158] (6) Coat the compressed microtablets with a seal coat solution.
[0159] (7) Filling the microtablets obtained in step (6) into hard gelatin capsules.
Embodiment 2
[0161]
[0162] method:
[0163] 1. Prepare a dry mixture of ritonavir, colloidal silicon dioxide and Eudragit.
[0164] 2. Extrude the dry mixture obtained in step (1) using a hot-melt extrusion technique.
[0165] 3. The extrudate obtained in step (2) is sized and sieved to form granules.
[0166] 4. Mix the granules obtained in step (3) with colloidal silicon dioxide and sugar.
Embodiment 3
[0168]
[0169] method:
[0170] 1. Prepare a dry mixture of ritonavir, colloidal silicon dioxide, sugar and Eudragit.
[0171] 2. Extrude the dry mixture obtained in step (1) using a hot-melt extrusion technique.
[0172] 3. The extrudate obtained in step (2) is sized and sieved to form granules.
[0173] 4. Add colloidal silicon dioxide and mix the granules obtained in step (3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com