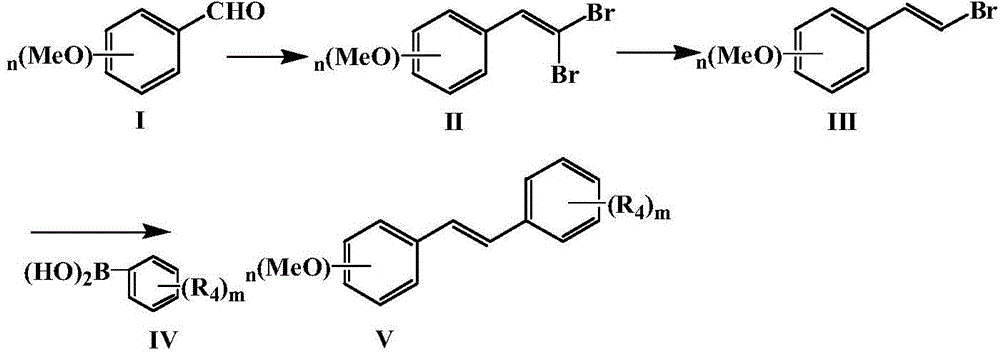
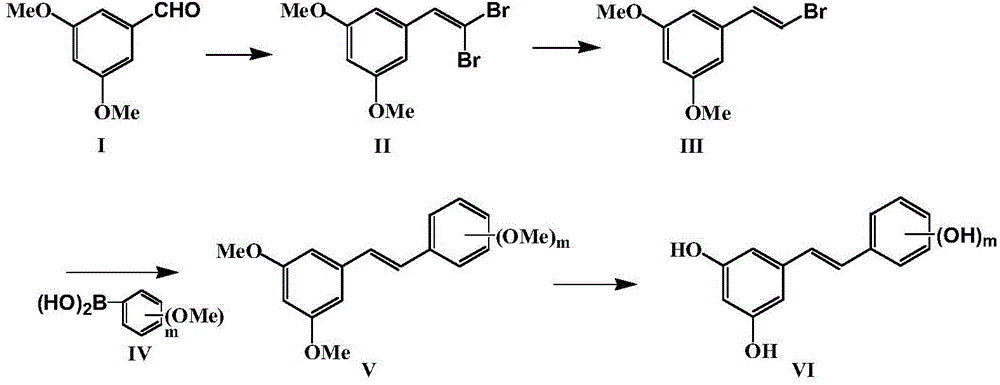
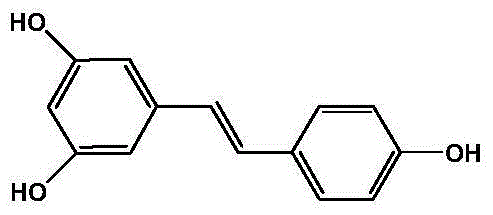
Method for preparing resveratrol and derivative of resveratrol
A technology of resveratrol and its derivatives, applied in the field of phytochemistry, can solve the problems of poor selectivity, harsh reaction conditions, cumbersome steps, etc., and achieve the effect of good stereoselectivity and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of resveratrol (trans-3,5,4'-trihydroxystilbene): (m=1)
[0037]
[0038] 1.Corey-Fuchs reaction: Synthesis of 1,1-dibromo-3′,5′-dimethoxystyrene
[0039]Get carbon tetrabromide (10.5g, 31mmol) and join in clean and dry 250mL reaction bottle, add the organic solvent methylene chloride of 100mL, add triphenylphosphine (16.4g, 62mmol) and 3,5-dimethoxy Benzaldehyde (2.5g, 15mmol) reacted completely under stirring at room temperature-20°C. After separation and drying, 4.5g of white solid was obtained with a yield of 92.7%.
[0040] The molar ratio of 3,5-dimethoxybenzaldehyde: carbon tetrabromide: triphenylphosphine is = 1: 2.06: 4.13
[0041] MP:73.7-74.5℃;IR(KBr):2997,1600,1456,1417,1337,1143,879,816,676,629cm -1 . 1 HNMR (CDCl 3 ): δ3.79(s,6H),6.45(t,J=2.3Hz,1H),6.68(dd,J=2.3Hz,2H),7.42(s,1H)ppm.
[0042] 2. Reduction reaction: Synthesis of 1-bromo-3′,5′-dimethoxystyrene
[0043] In a dry 100mL flask, add 1,1-dibromo-3′,5′-dimethoxysty...
Embodiment 2
[0053] Example 2: Preparation of Compound 2 (trans 3,5,2',4'-tetrahydroxystilbene): (m=2)
[0054]
[0055] 1.Corey-Fuchs reaction: Synthesis of 1,1-dibromo-3′,5′-dimethoxystyrene
[0056] Take carbon tetrabromide (10.5g, 31mmoL) and add it to a clean and dry 250mL reaction flask, add 100mL of organic solvent methylene chloride, add triphenylphosphine (16.4g, 62mmol) and 3,5-dimethoxy Benzaldehyde (2.5g, 15mmol) reacted completely under stirring at room temperature 20°C. After separation and drying, 4.5g of white solid was obtained with a yield of 92.7%.
[0057] The molar ratio of 3,5-dimethoxybenzaldehyde: carbon tetrabromide: triphenylphosphine is = 1: 2.06: 4.13
[0058] MP:73.7-74.5℃;IR(KBr):2997,1600,1456,1417,1337,1143,879,816,676,629cm -1 . 1 HNMR (CDCl 3 ): δ3.79(s,6H),6.45(t,J=2.3Hz,1H),6.68(dd,J=2.3Hz,2H),7.42(s,1H)ppm.
[0059] 2. Reduction reaction: Synthesis of 1-bromo-3′,5′-dimethoxystyrene
[0060] In a dry 100mL flask, add 1,1-dibromo-3′,5′-dimethoxys...
Embodiment 3
[0069] Example 3: Preparation of compound 3 (trans 3,5,3',4'-tetrahydroxystilbene): (m=2)
[0070]
[0071] 1.Corey-Fuchs reaction: Synthesis of 1,1-dibromo-3′,5′-dimethoxystyrene
[0072] Take carbon tetrabromide (5.3g, 15.5mmol) and join in a clean and dry 100mL reaction flask, add 30mL of chloroform, add triphenylphosphine (8.2g, 31mmol) and 3,5-dimethoxybenzene Formaldehyde (1.3g, 7.8mmol) was stirred and reacted at room temperature at 40°C. After complete separation and drying, 2.3g of white solid was obtained with a yield of 93%.
[0073]The molar ratio of 3,5-dimethoxybenzaldehyde: carbon tetrabromide: triphenylphosphine is =1:2:0.5.
[0074] MP:73.7-74.5℃;IR(KBr):2997,1600,1456,1417,1337,1143,879,816,676,629cm -1 . 1 HNMR (CDCl 3 ): δ3.79(s,6H),6.45(t,J=2.3Hz,1H),6.68(dd,J=2.3Hz,2H),7.42(s,1H)ppm.
[0075] 2. Reduction reaction: Synthesis of 1-bromo-3′,5′-dimethoxystyrene
[0076] In a dry 100 mL flask, add 1,1-dibromo-3′,5′-dimethoxystyrene (3 g, 9.2 mmol), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com