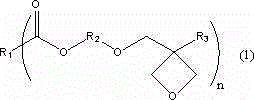
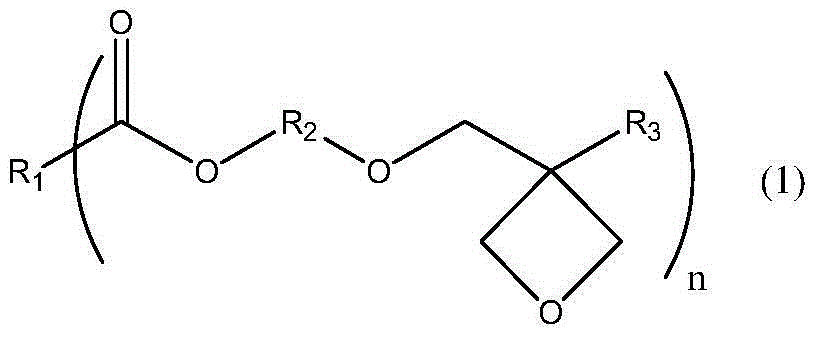
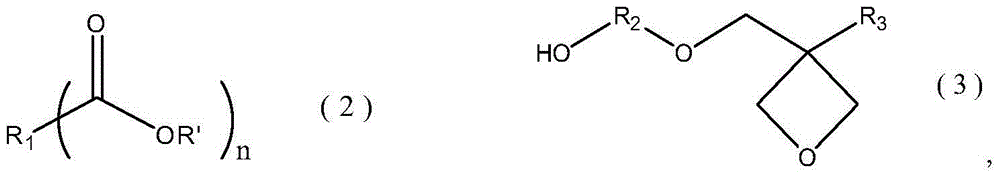
Ester compound containing oxetane group and preparation method thereof
A technology of heterocyclobutane group and ester compound, which is applied in the field of ester compound containing oxetane group and its preparation, can solve the problems of reducing production efficiency, etc., and achieve simple preparation, high yield and high cationic The effect of reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of compound 1
[0034]
[0035]In a glass flask with a volume of 1000 mL having a stirring device, a thermometer, a 60 cm packed tower and a rectifying head, add 160 g (1.0 mol) of raw material 1 and 500 g (5.0 mol) of methyl methacrylate, keep reflux at 110 ° C, and adjust The reflux ratio was 3:1. After the moisture in the system dropped below 500ppm, 6.6g of tetraisopropyl titanate catalyst (accounting for 1% of the total feeding amount) was added, and the reflux was continued for 3 hours. After the reaction, cool down to 70°C, add 25g of water, keep warm at 70°C for half an hour, destroy the catalyst, filter, and concentrate the filtrate to obtain 216g colorless liquid (isolation yield of raw material 1 benchmark: 95%)
[0036] The structure of the product compound 1 was obtained by mass spectrometry and 1 H-NMR confirmed.
[0037] MS(m / e): 229(M+1)
[0038] 1 H-NMR (CDCl 3 , δ(ppm)): 0.96(3H), 1.25(2H), 1.93(3H), 3.65(2H), 4.32(2H), 4.65(4H), 5.58...
Embodiment 2
[0040] Synthesis of compound 2
[0041]
[0042] In a glass flask with a volume of 1000 mL having a stirring device, a thermometer, a 60 cm packed tower and a rectifying head, add 204 g (1.0 mol) of raw material 2, and refer to Example 1 for the remaining operations. After concentration, 261 g of a colorless transparent liquid was obtained (isolation yield based on raw material 2: 96%).
[0043] The structure of the product was confirmed by the following physical property values.
[0044] MS(m / e): 273(M+1)
[0045] 1 H-NMR (CDCl 3 , δ(ppm)): 0.96(3H), 1.25(2H), 1.93(3H), 3.29(2H), 3.54(4H), 3.65(2H), 4.32(2H), 4.65(4H), 5.58(2H ), 6.15(2H).
Embodiment 3
[0047] Synthesis of compound 3
[0048]
[0049] In a glass flask with a volume of 1000 mL having a stirring device, a thermometer, a 60 cm packed tower and a rectifying head, add 248 g (1.0 mol) of raw material 3, and refer to Example 1 for the remaining operations. Concentration gave 294 g of a colorless transparent liquid (isolation yield based on raw material 3: 93%).
[0050] The structure of the product was confirmed by the following physical property values.
[0051] MS(m / e): 317(M+1)
[0052] 1 H-NMR (CDCl 3 , δ(ppm)): 0.96(3H), 1.25(2H), 1.93(3H), 3.29(2H), 3.54(8H), 3.65(2H), 4.32(2H), 4.65(4H), 5.58(2H ), 6.15(2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com