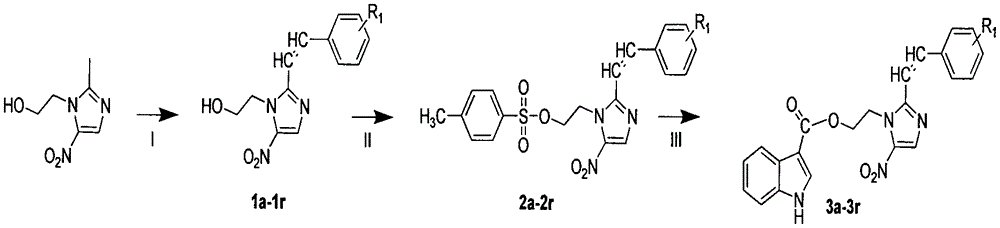
Synthesis and bioactivity evaluation of 1-indoleacetic acid-5-nitroimidazole-containing derivative thereof
A technology of indoleacetic acid and nitroimidazole, which can be used in drug combination, organic chemistry, antitumor drugs, etc., can solve problems such as easy isomerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
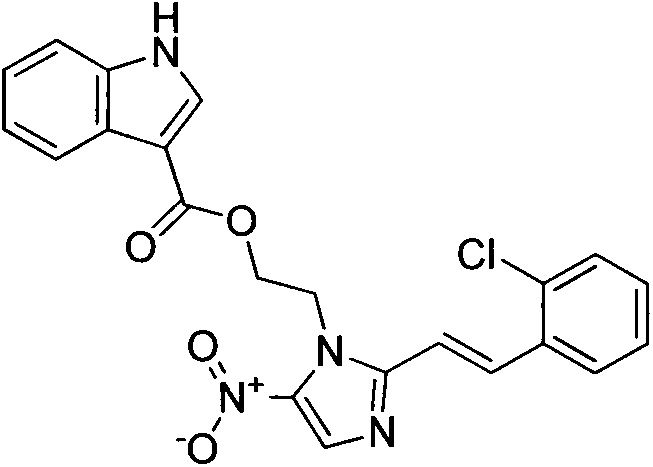
[0013] Example 1: Preparation of (E)-2-(2-(2-chlorostyryl)-5-nitroimidazole)ethanolindole-3-carboxylic acid (3a)
[0014]
[0015] Add compound 2 (5mmol) into a 25mL round bottom flask containing 15mL DMF, stir until dissolved, then add isoquinoline-3-carboxylic acid (5mmol), dissolve and add K 2 CO 3 (5 mmol) as catalyst. The reaction was carried out under reflux overnight (TLC followed the reaction until there was little or no at least one raw material), and the crude product was obtained, and the product was purified by column chromatography with ethyl acetate / hexane ratio of 1:9 to obtain the target compound. product. Yield 64.5%.m.p.189~191℃; 1 H NMR (DMSO-d 6 , 300MHz) δ: 11.71(s, 1H), 8.23(s, 1H), 7.95-7.69(m, 4H), 7.58(t, J=7.9Hz, 2H), 7.33(t, J=7.5Hz, 2H ), 7.14-7.02(m, 3H), 5.02(t, J=4.3Hz, 2H), 4.65(t, J=4.4Hz, 2H).
Embodiment 2
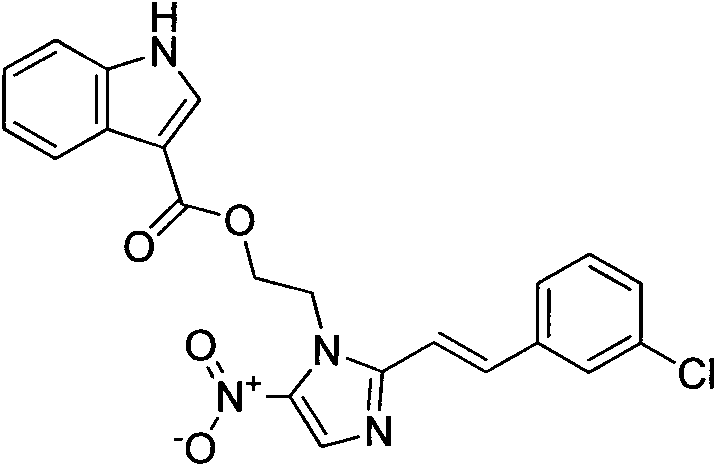
[0016] Example 2: Preparation of (E)-2-(2-(3-chlorostyryl)-5-nitroimidazole)ethanolindole-3-carboxylic acid (3b)
[0017]
[0018] The preparation method is the same as in Example 2. (E)-2-(2-(3-chlorostyryl)-5-nitroimidazole)ethanol-4-methylbenzenesulfonic acid is used instead of (E)-2-(2- (2-chlorostyryl)-5-nitroimidazole)ethanol-4-methylbenzenesulfonic acid to obtain the title compound. Yield yield 66.5%.m.p.188~190℃; 1 H NMR (DMSO-d 6 , 300MHz) δ: 11.68(s, 1H), 8.26(t, J=7.9Hz 2H), 8.06(d, J=8.4Hz, 2H), 7.76(t, J=8.4Hz, 4H), 7.29(t , J=8.5Hz, 1H), 7.19-7.00(m, 3H), 5.03(t, J=4.3Hz, 2H), 4.65(t, J=4.4Hz, 2H)
Embodiment 3
[0019] Example 3: Preparation of (E)-2-(2-(4-chlorostyryl)-5-nitroimidazole)ethanolindole-3-carboxylic acid (3c)
[0020]
[0021] The preparation method is the same as in Example 2. (E)-2-(2-(4-chlorostyryl)-5-nitroimidazole)ethanol-4-methylbenzenesulfonic acid is used instead of (E)-2-(2- (2-chlorostyryl)-5-nitroimidazole)ethanol-4-methylbenzenesulfonic acid to obtain the title compound. Yield 67.5%.m.p.188~189℃; 1 H NMR (DMSO-d 6 , 300MHz) δ: 11.77(s, 1H), 8.22(s, 1H), 7.89(t, J=10.1Hz, 2H), 7.77(d, J=2.7Hz, 1H), 7.69-7.59(m, 3H ), 7.44-7.31(m, 3H), 7.14-7.02(m, 2H), 4.99(t, J=4.4Hz, 2H), 4.62(t, J=4.3Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com