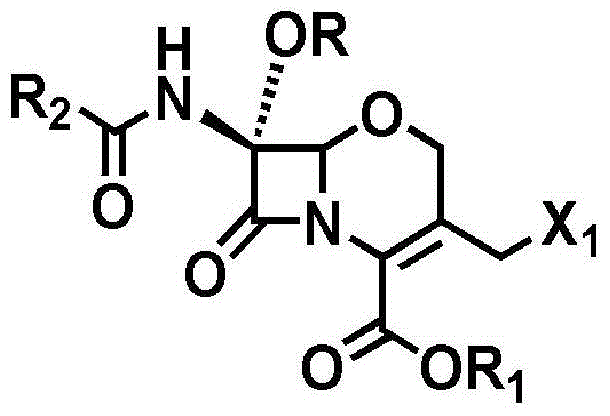
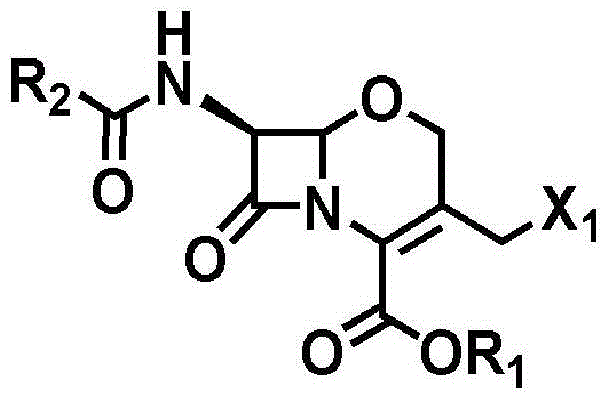
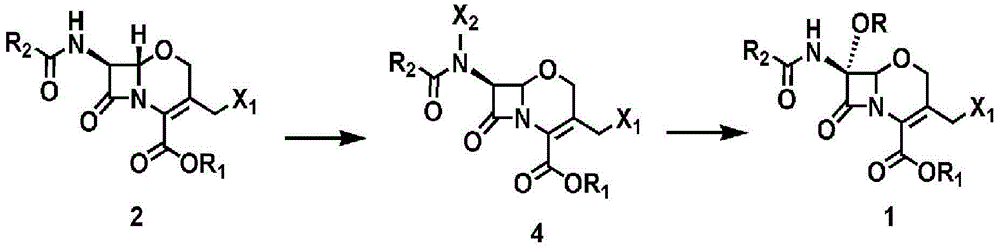
Novel method for preparing 1-oxacephalosporin derivative
A technology of oxacephalosporins and manufacturing methods, applied in the new manufacturing field of 1-oxacephalosporin derivatives, which can solve the problems of low yield, high light and expensive photoreaction equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] [Example 1] (6R,7R)-7-(difluoromethylthio)acetamido)-3-(chloromethyl)-7-methoxy-8-oxo-5-oxo Production of diphenylmethyl hetero-1-aza-bicyclo[4,2,0]oct-2-ene-2-carboxylate
[0082] Step 1. (6R,7R)-7-(N-chloro-4-methylbenzamido)-3-(chloromethyl)-8-oxo-5-oxa-1-azabicyclo[ 4.2.0] Synthesis of oct-2-ene-2-carboxylic acid benzhydryl ester
[0083] A. Dichloromethane 1804mL, (6R,7R)-7-(4-methylbenzamido)-3-(chloromethyl)-8-oxo-5-oxa-1-aza- 180.4 g of diphenylmethyl bicyclo[4.2.0]oct-2-ene-2-carboxylate was charged into the reactor, and the reaction solution was cooled to 0°C. After adding 35.7 g of 2-picoline and cooling to -55°C, 122.7 g of trichloroisocyanuric acid was added dropwise. At this time, after the temperature of the reaction solution was reacted at -55±5°C for 30 minutes, the target compound, namely (6R,7R)-7-(N-chloro-4-methylbenzamido)-3- A solution of diphenylmethyl (chloromethyl)-8-oxo-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate.
[0084] B. Dichl...
Embodiment 2
[0099] [Example 2] (7R)-3-((1-methyl-1H-tetrazol-5-ylthio)methyl)-7-(4-methylbenzamido)-7-methyl Production of Diphenylmethyl Oxy-8-oxo-5-oxa-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate
[0100] Step 1. (6R,7R)-7-(N-chloro-4-methylbenzamido)-3-(chloromethyl)-8-oxo-5-oxa-1-azabicyclo[ 4.2.0] Synthesis of oct-2-ene-2-carboxylate diphenylmethyl ester
[0101] A. Dichloromethane 1804mL, (6R,7R)-7-(4-methylbenzamido)-3-(chloromethyl)-8-oxo-5-oxa-1-aza- 180.4 g of diphenylmethyl bicyclo[4.2.0]oct-2-ene-2-carboxylate was charged into the reactor, and the reaction solution was cooled to 0°C. After adding 35.7 g of 2-picoline and cooling to -55 degreeC, 122.7 g of trichloroisocyanuric acid was dripped. At this time, after the temperature of the reaction solution was reacted at -50±5°C for 30 minutes, the target compound, namely (6R,7R)-7-(N-chloro-4-methylbenzamido)-3- A solution of diphenylmethyl (chloromethyl)-8-oxo-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate.
[0102] B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com