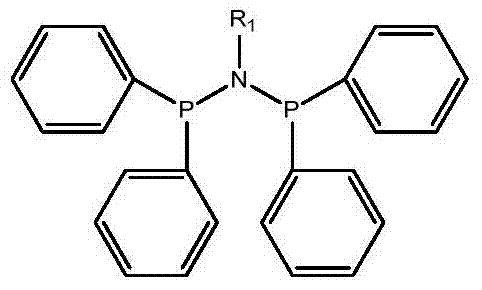
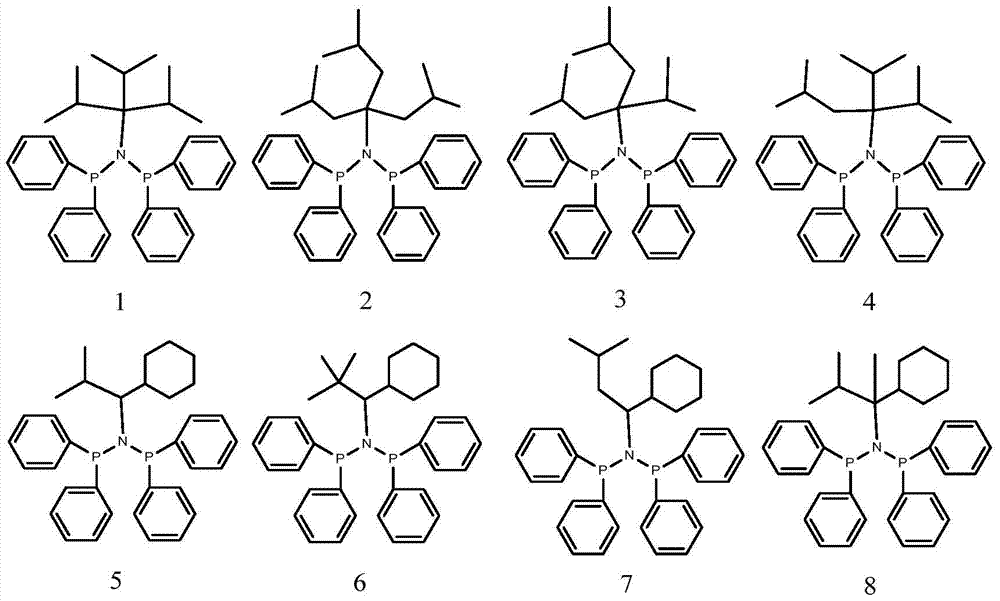
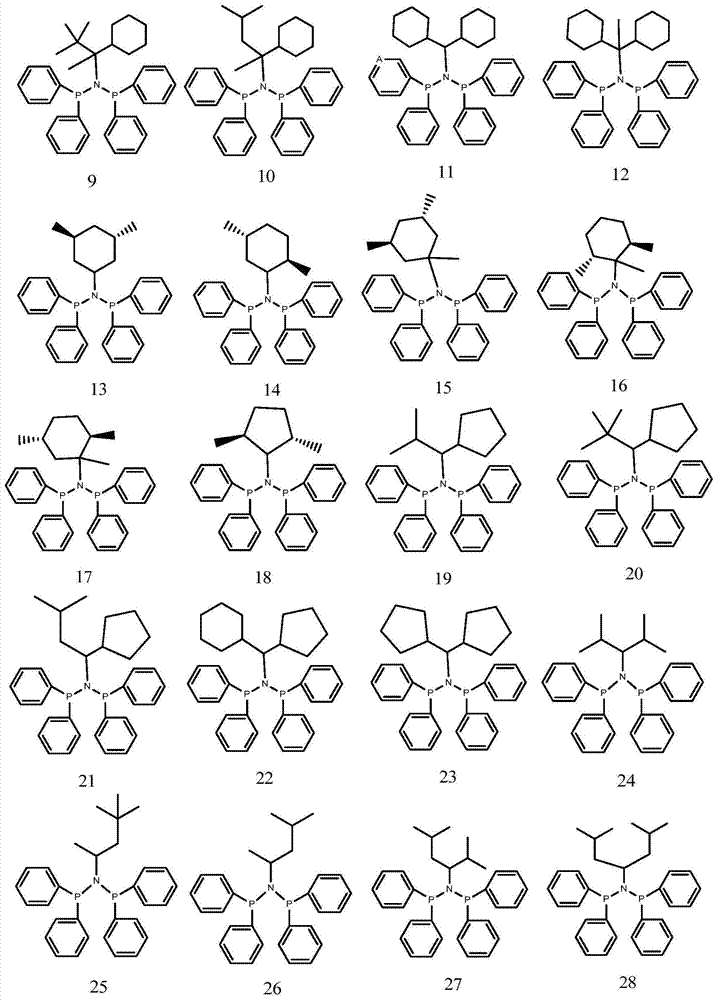
A highly selective ethylene trimerization and tetramerization catalyst system and its application method
A technology of ethylene trimerization and high selectivity, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Impact and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] 1 Preparation of Ligand 5
[0077] At 0°C, diphenylphosphine chloride and the corresponding (isopropylcyclohexyl)methylamine were added into the dichloromethane solution at a molar ratio of 2:1 and stirred. After stirring for 40 minutes, continue to stir overnight at room temperature. Amine hydrochloride produced in the reaction was removed by filtration. The product was obtained after recrystallization with a yield of 81%.
[0078] 2 ethylene oligomerization reaction
[0079] Ethylene oligomerization was carried out in a 300mL autoclave. Before oligomerization started, the reactor was replaced with nitrogen three times and with ethylene twice. Then add solvent toluene into the autoclave, stir under ethylene atmosphere, and heat to set temperature. The predetermined amount of MAO, ligand and CrCl 3 (THF) 3 Add to the autoclave and stir for 1 min. Feed ethylene into the reactor and adjust the pressure in the reactor to reach the set value, and the reaction starts....
Embodiment 2
[0081] 1 Preparation of Ligand 7
[0082] At 0°C, diphenylphosphine chloride and the corresponding (isobutylcyclohexyl)methylamine were added to the dichloromethane solution at a molar ratio of 2:1 and stirred. After stirring for 40 minutes, continue to stir overnight at room temperature. Amine hydrochloride produced in the reaction was removed by filtration. The product was obtained after recrystallization with a yield of 76%.
[0083] 2 ethylene oligomerization reaction
[0084] Ethylene oligomerization was carried out in a 300mL autoclave. Before the oligomerization starts, the reactor is replaced with nitrogen and ethylene to keep the oxygen value of the water in the reactor low. Then add solvent toluene into the autoclave, stir under ethylene atmosphere, and heat to set temperature. A predetermined amount of MAO, ligand and Cr(acac) 3 Add to the autoclave and stir for 1 min. Feed ethylene into the reactor and adjust the pressure in the reactor to reach the set value...
Embodiment 3
[0086] 1 Preparation of Ligands 17
[0087] At 0°C, add chlorinated diphenylphosphine and the corresponding (1,2,5-trimethyl)cyclohexylamine into the dichloromethane solution at a molar ratio of 2:1 and stir. After stirring for 40 minutes, continue to Stir overnight. Amine hydrochloride produced in the reaction was removed by filtration. The product was obtained after recrystallization with a yield of 70%.
[0088] 2 ethylene oligomerization reaction
[0089] Ethylene oligomerization was carried out in a 300mL autoclave. Before the oligomerization starts, the reactor is replaced with nitrogen and ethylene to keep the oxygen value of the water in the reactor low. Then add solvent toluene into the autoclave, stir under ethylene atmosphere, and heat to set temperature. Add predetermined amount of MAO, ligand and chromium(III) 2-ethylhexanoate into the autoclave and stir for 1 min. Feed ethylene into the reactor and adjust the pressure in the reactor to reach the set value, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com