Aromatic polyamide resin containing hydroxyl group and allyl group, preparation method and application thereof
An aromatic polyamide and allyloxy technology, which is applied in the fields of hydroxyl and allyl aromatic polyamide resin and its preparation and application, can solve the problems of difficult interaction, high glass transition temperature, poor dyeing ability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
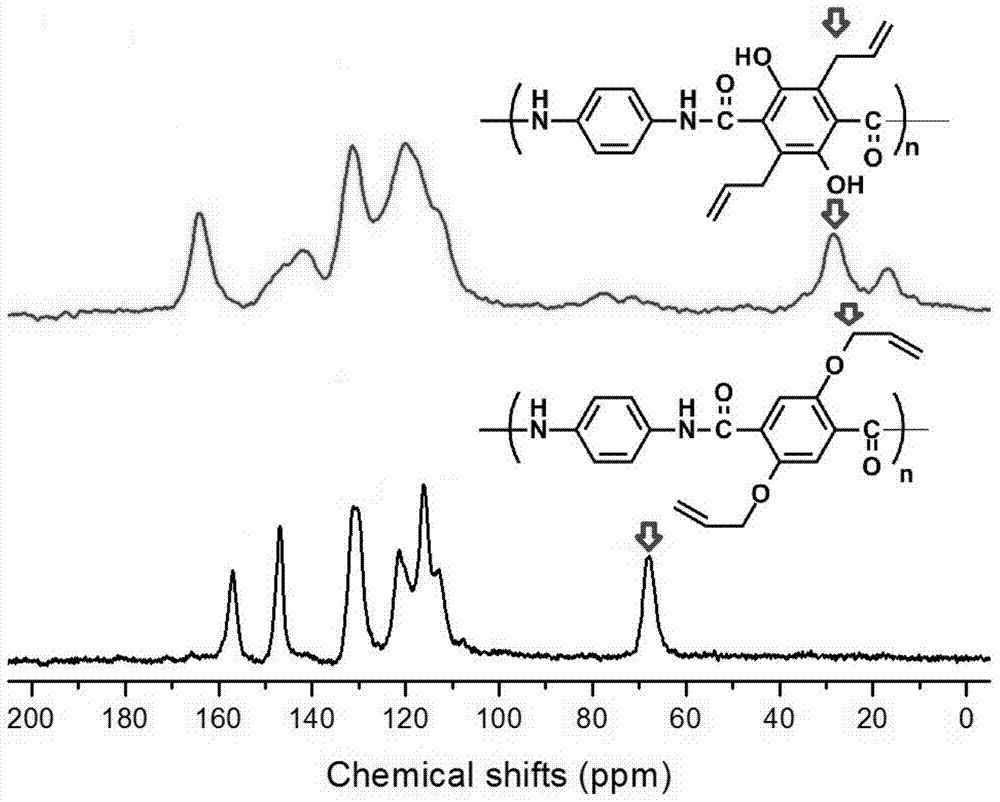
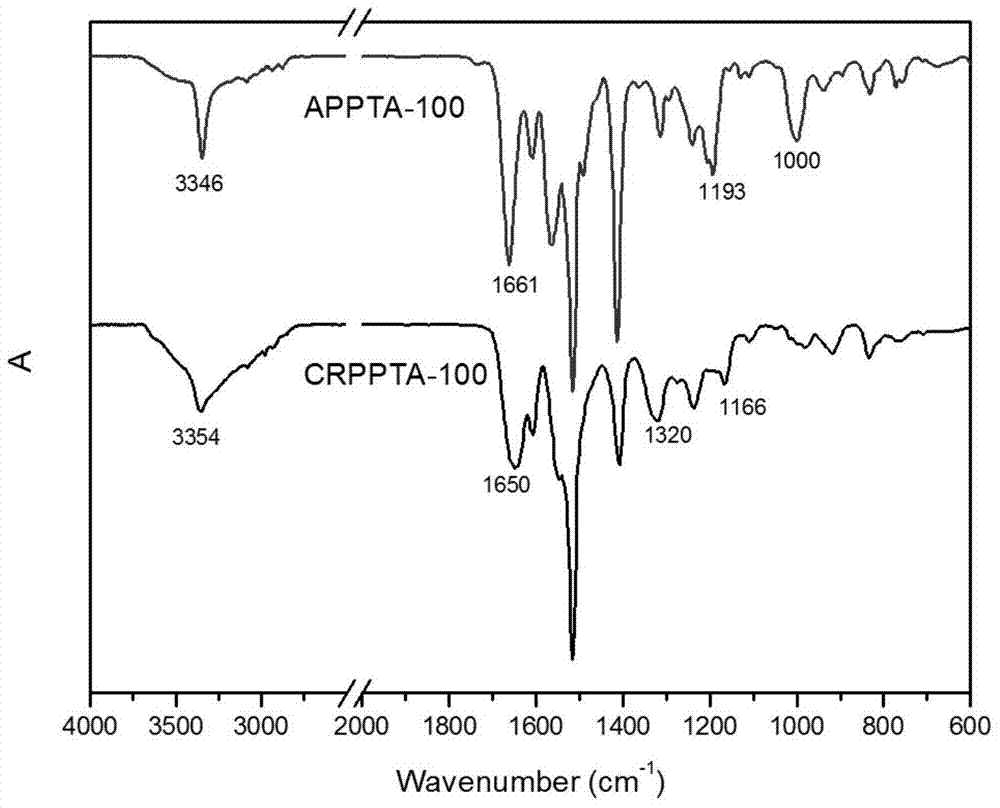
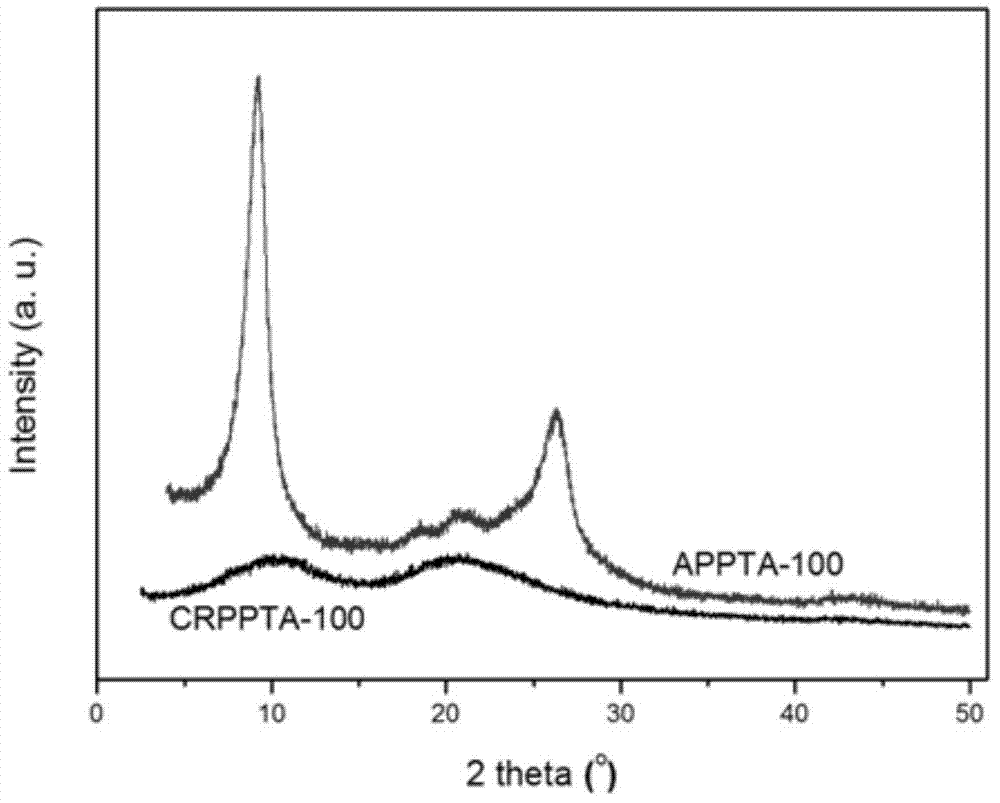
[0032] Put the raw material APPTA-100 (molecular weight: 9500) into the reaction tube, heat it to 190°C under the protection of nitrogen, and carry out the Claisen rearrangement reaction at constant temperature for 10 hours, and then cool the reactant to room temperature to obtain the product CRPPTA-100 (Molecular weight is 9500).
Embodiment 2
[0034]
[0035] The raw material APPTA-50 (molecular weight: 10400) was put into the reaction tube, heated to 190°C under the protection of nitrogen, and the Claisen rearrangement reaction was carried out at constant temperature for 8 hours. Then the reactant was cooled to room temperature to obtain the product CRPPTA-50 (molecular weight 10400).
Embodiment 3
[0037]
[0038] The raw material APPTA-25 (molecular weight: 7600) was put into a reaction tube, heated to 190°C under the protection of nitrogen, and the Claisen rearrangement reaction was carried out at constant temperature for 8 hours. Then the reactant was cooled to room temperature to obtain the product CRPPTA-25 (molecular weight 7600).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com