CTLA4 fusion proteins for the treatment of diabetes
A technology of fusion protein and diabetes, which is applied in the field of prevention or delay of type 1 diabetes progression and treatment, and can solve problems such as no cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
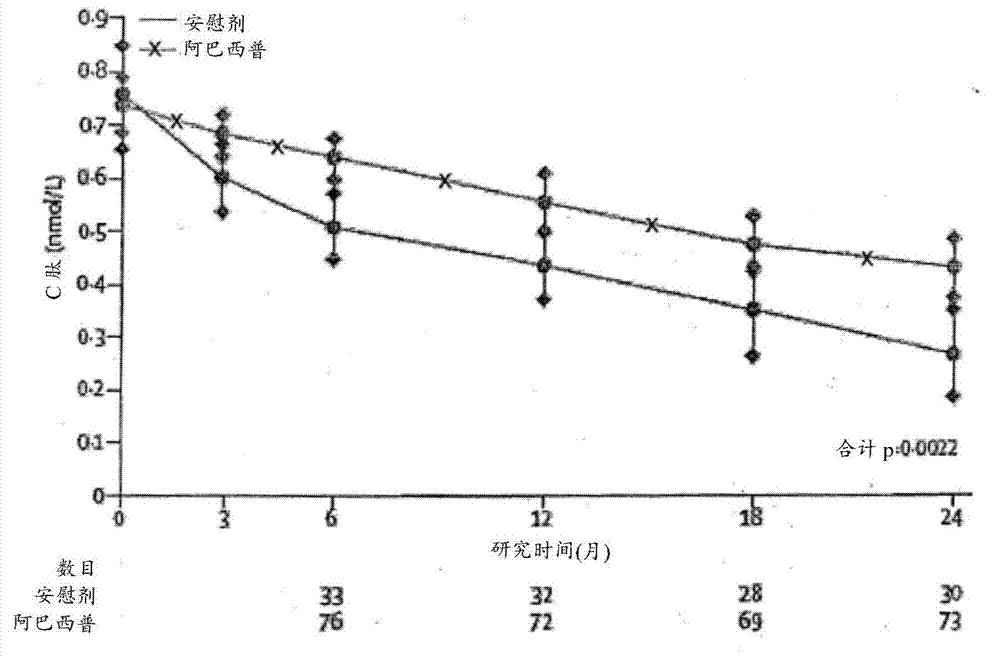
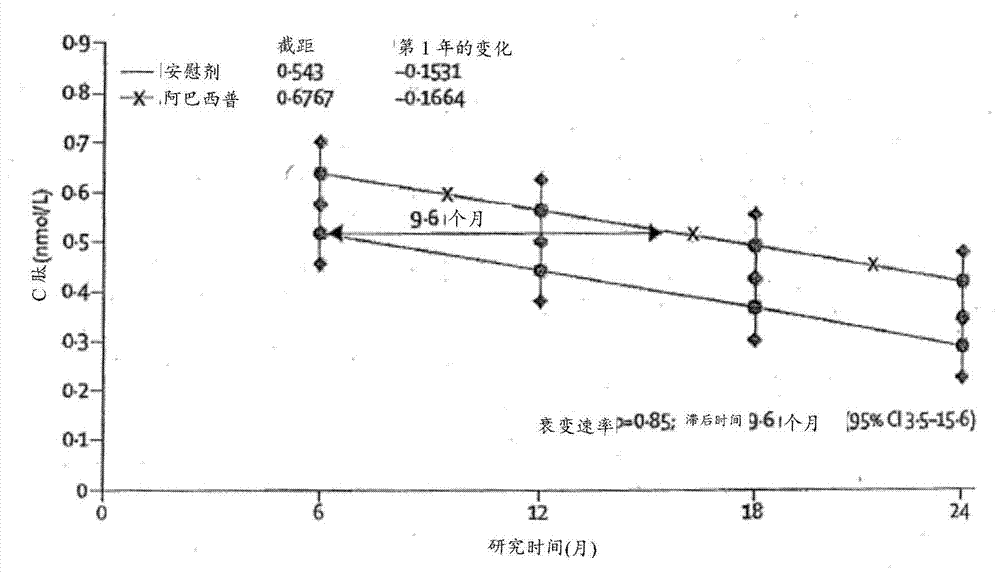
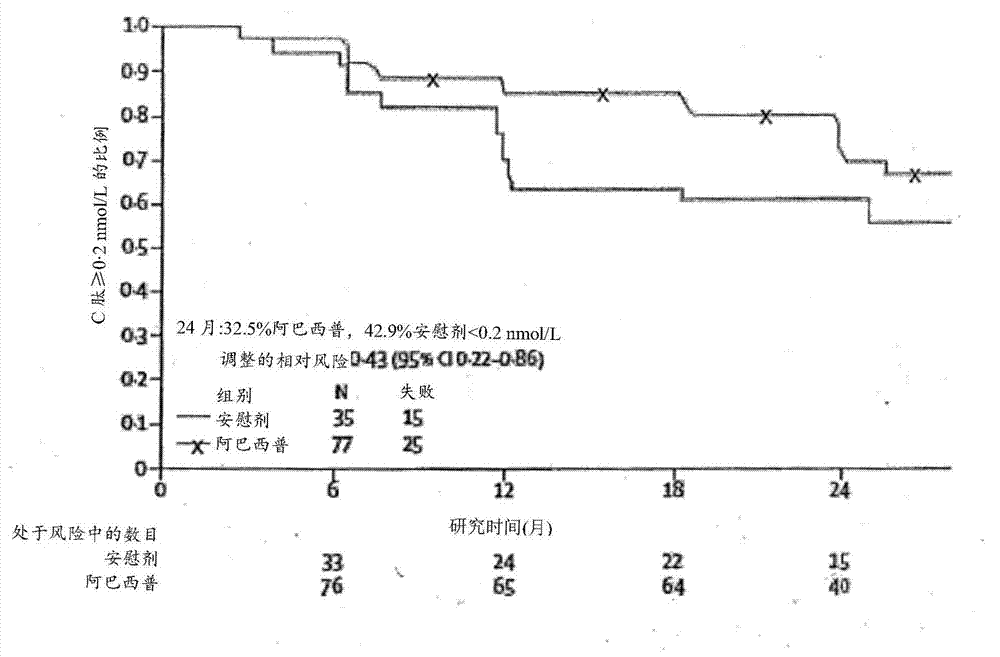
[0072] Example 1 - Study Design and Patients
[0073] Patients (6-45 years old) diagnosed with type 1 diabetes within the past 100 days were screened in parallel for this study. If patient has at least one diabetes-associated autoantibody (micromeasure insulin antibody [if duration of insulin therapy is less than 7 days]; glutamic acid decarboxylase-65 [GAD-65] antibody; islet cell antigen-512 [ICA-512] or islet cell autoantibodies) and have a stimulated C of 0.2 nmol / L or greater measured during the Mixed Meal Tolerance Test (MMTT) completed at least 21 days after diagnosis of diabetes and within the 37-day randomization period peptide concentration, then the patient is eligible to participate in the study.
[0074] Persons whose blood samples screened positive for serum antibodies to hepatitis B surface antigen, hepatitis C, or HIV were excluded from the study. Samples were also tested for Epstein-Barr virus (EBV). Individuals with evidence of active EBV infection at scre...
Embodiment 2-
[0081] Example 2 - Procedure
[0082] Abatacept (Orencia, Bristol-Myers Squibb, Princeton, NJ, USA) was given on days 1, 14, and 28, then every 28 days, with the last dose on day 700 (total of 27 doses), given The form is a 30-minute intravenous infusion at a dose of 10 mg / kg (maximum 1000 mg per dose) in 100 mL of 0.9% sodium chloride infusion. Standard saline infusion was used as placebo. Patients did not receive any premedication.
[0083] All patients received intensive diabetes management. The goal is to achieve intensive glycemic control as recommended by the American Diabetes Association. (American Diabetes Association. Diabetes Care 2011;33(Suppl 1):S11–61.) Patients used multiple daily insulin injections or an insulin pump. Blood glucose monitoring was performed by frequent daily blood glucose monitoring. Non-insulin drugs that affect blood sugar control are not allowed.
[0084]Central analysis of blood samples. C-peptide concentrations from frozen plasma were...
Embodiment 3
[0085] Example 3 - Statistical Analysis
[0086] Spotfire S+8.1 statistical analysis software was used for all analyses. A sample size of 108 participants was planned to provide 85% power to detect a 50% increase in geometric mean C-peptide relative to placebo using a 0.05 level (one-sided) test with a follow-up loss of 10% and assigned treatment compared to 2:1 compared to controls (based on estimated mean of 0.248 and SD of 0.179 on the transformed scale). All analyzes were based on prespecified intention-to-treat cohorts with known measurements. Missing values were assumed to be missing at random. The p-values associated with the intention-to-treat comparisons of the primary and secondary endpoints were two-sided, but the trial design was based on a one-sided hypothesis test. An interim analysis of the endpoint treatment effect was completed and reported once to the Data and Safety Monitoring Committee according to the Lan and DeMets method with O'Brien-Fleming bound...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap