Cefathiamidine composition
A cefathiamidine and composition technology, which is applied in the directions of inactive components of polymer compounds, antibacterial drugs, organic active components, etc., can solve the problems of long-term storage, easy oxidative deterioration, short antibacterial time, etc., and achieves hard oxidative deterioration. , long antibacterial time, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
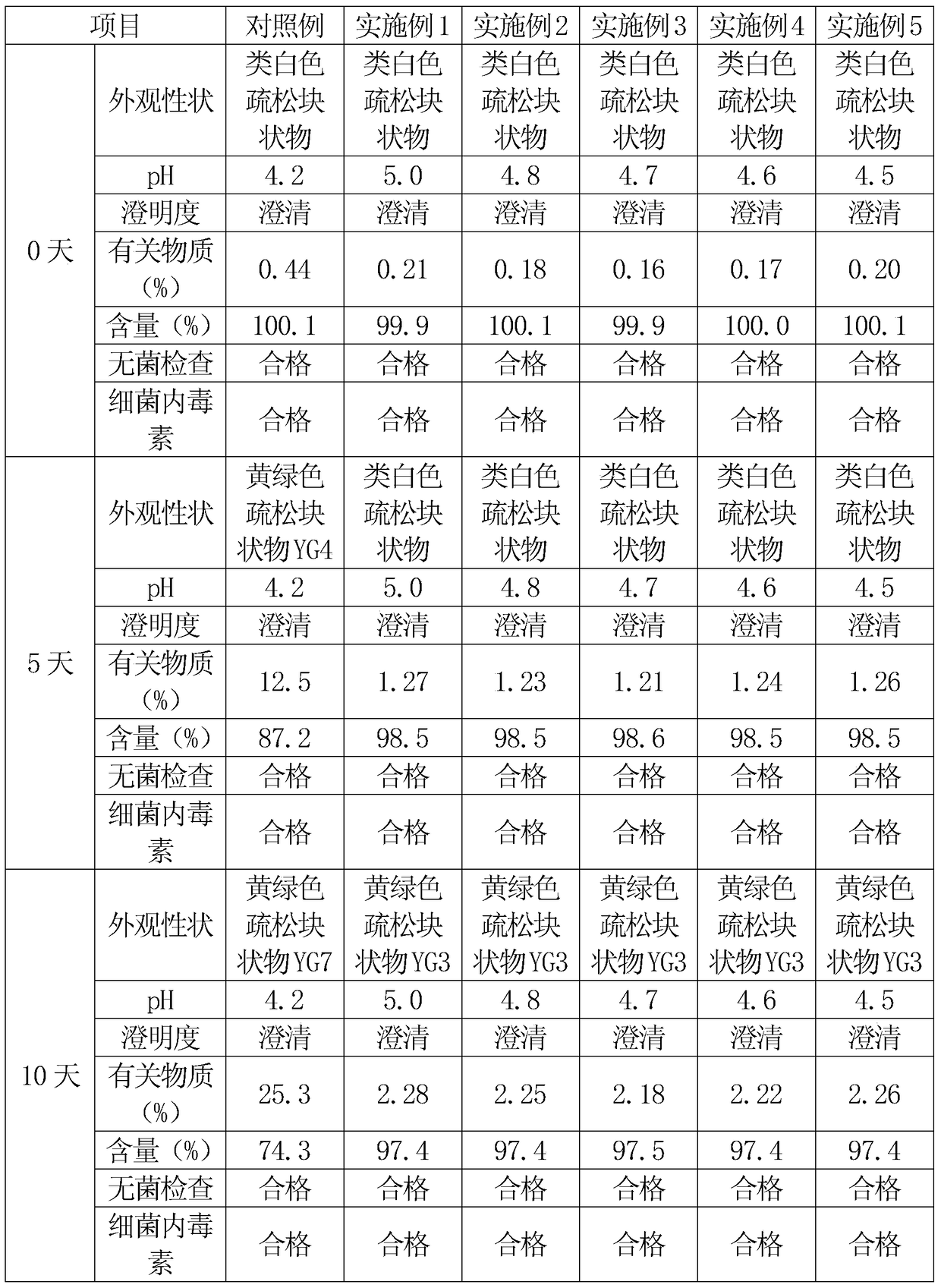
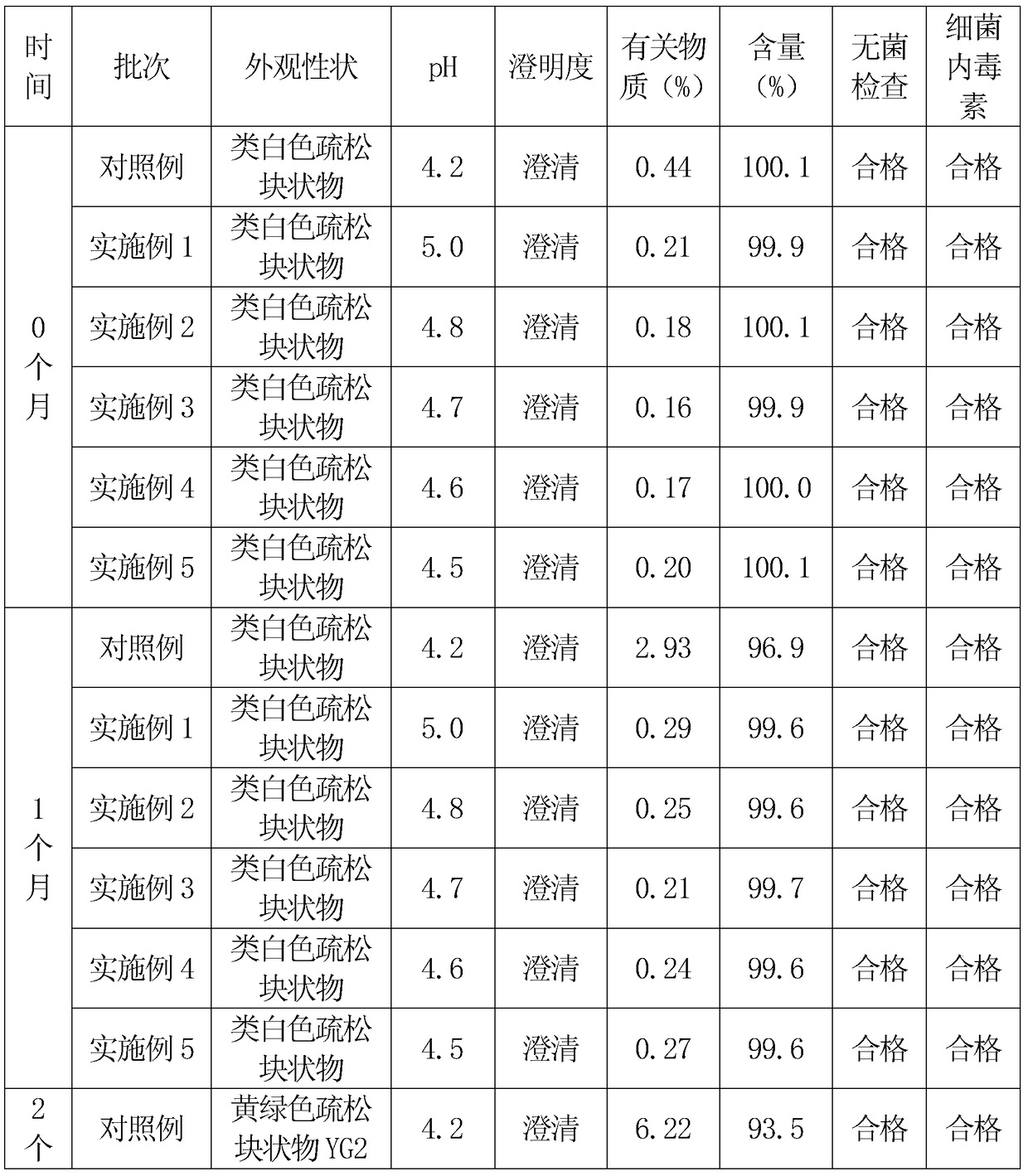
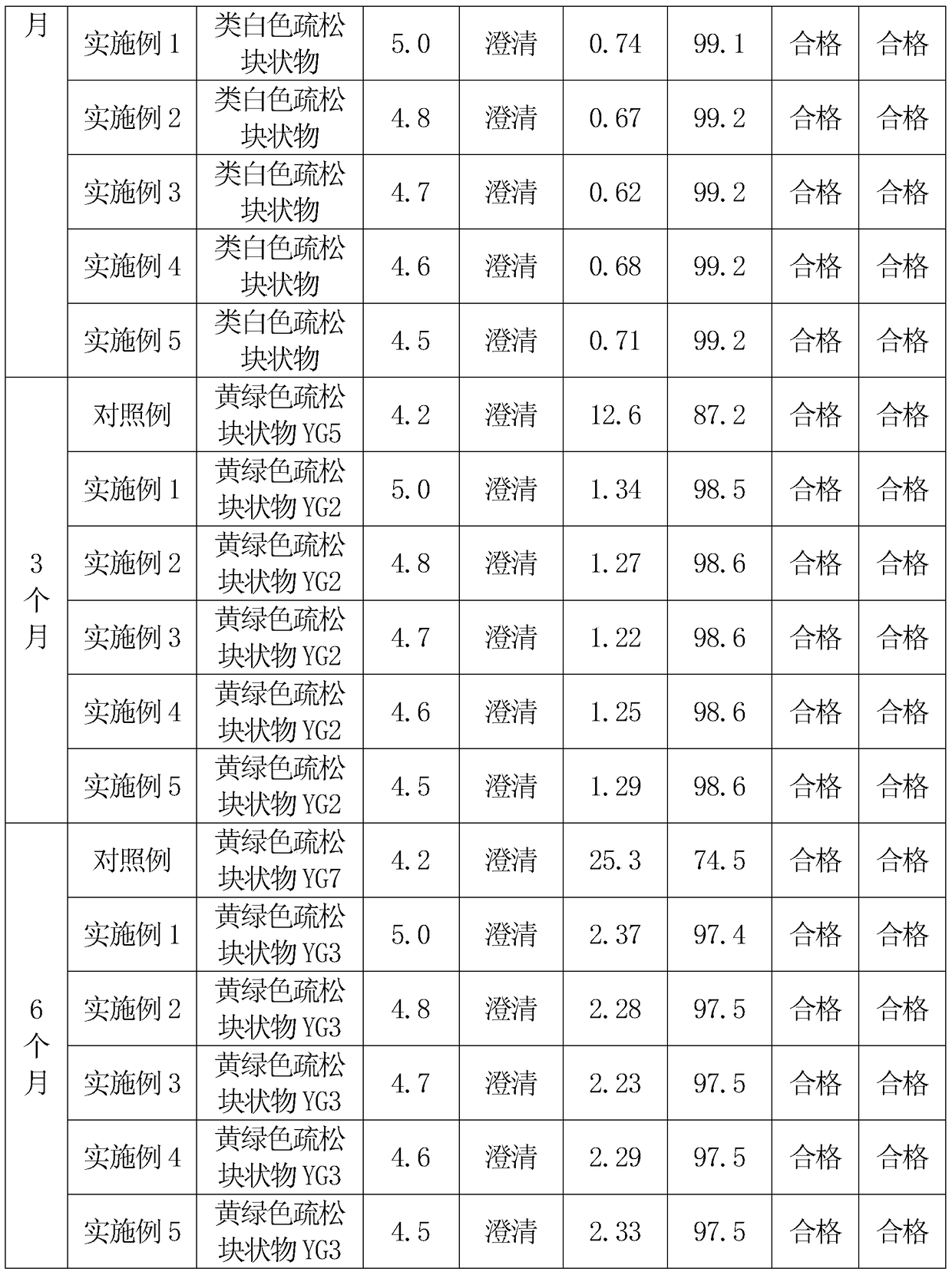
[0025] First, 250 grams of hydroxypropyl-β-cyclodextrin is dissolved in 1500 grams of water for injection to obtain a hydroxypropyl-β-cyclodextrin solution. Then add 50 grams of cefathiamidine into the hydroxypropyl-β-cyclodextrin solution, heat to 40°C, and heat and stir at 40°C for 4 hours for inclusion, after the inclusion is completed, the mixture is cooled to room temperature and filtered After obtaining the filtrate.
[0026] 15 grams of low-molecular-weight dextran was heated and dissolved with 45 grams of water for injection to obtain a low-molecular-weight dextran solution, cooled to room temperature, and filtered. Add the low-molecular-weight dextran solution into the inclusion solution of hydroxypropyl-β-cyclodextrin and cefathiamidine, stir to dissolve, then add 5 grams of vitamin C, stir to dissolve, and adjust the pH value with a 5% citric acid solution to 5.0, finally add water for injection to 2000ml, stir evenly, and then filter and sterilize with a 0.22μm mi...
Embodiment 2
[0028] Firstly, 450 grams of hydroxypropyl-β-cyclodextrin is dissolved with 2600 grams of water for injection to obtain a hydroxypropyl-β-cyclodextrin solution. Then add 100 grams of cefathiamidine into the hydroxypropyl-β-cyclodextrin solution, heat to 45°C, heat and stir at 45°C for 3.5 hours for inclusion, after the inclusion, cool the mixture to room temperature, filter After obtaining the filtrate.
[0029] Heat and dissolve 25 grams of low-molecular-weight dextran with 80 grams of water for injection to obtain a low-molecular-weight dextran solution, cool to room temperature, and filter. Add the low-molecular-weight dextran solution into the inclusion solution of hydroxypropyl-β-cyclodextrin and cefathiamidine, stir to dissolve, then add 8 grams of vitamin C, stir to dissolve, and adjust the pH value with a 6% citric acid solution To 4.8, finally add water for injection to 3500ml, stir evenly, and then filter and sterilize with a 0.22μm microporous membrane, and fill it...
Embodiment 3
[0031] First, 800 grams of hydroxypropyl-β-cyclodextrin is dissolved with 4400 grams of water for injection to obtain a hydroxypropyl-β-cyclodextrin solution. Then add 200 grams of cefathiamidine into the hydroxypropyl-β-cyclodextrin solution, heat to 50°C, and heat and stir at 50°C for 3 hours to carry out inclusion. After the inclusion is completed, the mixture is cooled to room temperature and filtered. After obtaining the filtrate.
[0032] 40 grams of low-molecular-weight dextran was heated and dissolved with 160 grams of water for injection to obtain a low-molecular-weight dextran solution, cooled to room temperature, and filtered. Add the low-molecular-weight dextran solution into the inclusion solution of hydroxypropyl-β-cyclodextrin and cefathiamidine, stir to dissolve, then add 12 grams of vitamin C, stir to dissolve, and adjust the pH value with a 7% citric acid solution To 4.7, finally add water for injection to 6000ml, stir evenly, and then filter and sterilize w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com