Heterozygote of adjacent naphthoquinone and tetrazol-pyrimidine and synthetic method thereof
A technology of tetrazolium and aminotetrazolium, which is applied in the field of hybrids and achieves the effects of easy availability of raw materials, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
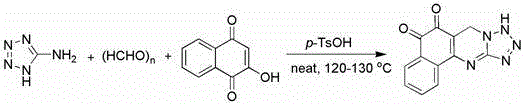
[0019] Put 0.85g of 5-aminotetrazole, 0.6g of paraformaldehyde, 1.74g of 2-hydroxy-1,4-naphthoquinone and 0.15g of p-toluenesulfonic acid together in a 25ml reaction flask and mix well, heat and stir, the temperature Controlled at 120°C, reacted for 1 hour. The reaction mixture was dissolved in 25 ml of dichloromethane, washed twice with 25 ml of water, dried over anhydrous sodium sulfate, evaporated to remove the solvent, and recrystallized with ethanol to obtain the corresponding yellow product 4,6-dihydro-benzo[ h ]tetrazolium[5,1- b ] Quinazoline-7,8-dione 1.92 g, productive rate is 76%.
[0020] After testing, molecular formula: C 12 h 7 N 5 o 2 , Molecular weight: 253.06, Appearance: Yellow solid, Melting point: >400℃;
[0021] IR (KBr): v 1672, 1656, 1589, 1573, 1308, 1072, 949, 720 cm -1 ;
[0022] 1 H NMR (400 MHz, DMSO- d 6 ) δ : 11.79 (s, 1H), 8.05 (dd, 1H, , J = 7.2, 17.6 Hz) , 7.93-7.84 (m, 2H), 5.39 (s, 2H);
[0023] 13 C NMR (100 MHz, DMSO- d...
Embodiment 2
[0026] Put 4.25g of 5-aminotetrazole, 3.3g of paraformaldehyde, 8.7g of 2-hydroxy-1,4-naphthoquinone and 0.75g of p-toluenesulfonic acid together in a 100ml reaction flask and mix well, heat and stir, the temperature Controlled at 125°C, after 2 hours of reaction. The reaction mixture was dissolved in 150 ml of dichloromethane, washed twice with 150 ml of water, dried over anhydrous sodium sulfate, evaporated to remove the solvent, and recrystallized with ethanol to obtain the corresponding yellow product 4,6-dihydro-benzo[ h ]tetrazolium[5,1- b ] Quinazoline-7,8-dione 10.21 g, productive rate is 81%.
Embodiment 3
[0028] Put 8.5g of 5-aminotetrazole, 7.5g of paraformaldehyde, 17.4g of 2-hydroxy-1,4-naphthoquinone and 1.5g of p-toluenesulfonic acid together in a 250ml reaction flask and mix evenly, heat and stir, the temperature Controlled at 130°C, reacted for 3 hours. The reaction mixture was dissolved in 300 ml of dichloromethane, washed twice with 300 ml of water, dried over anhydrous sodium sulfate, evaporated to remove the solvent, and recrystallized with ethanol to obtain the corresponding yellow product 4,6-dihydro-benzo[ h ]tetrazolium[5,1- b ] Quinazoline-7,8-dione 18.98 g, productive rate is 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com