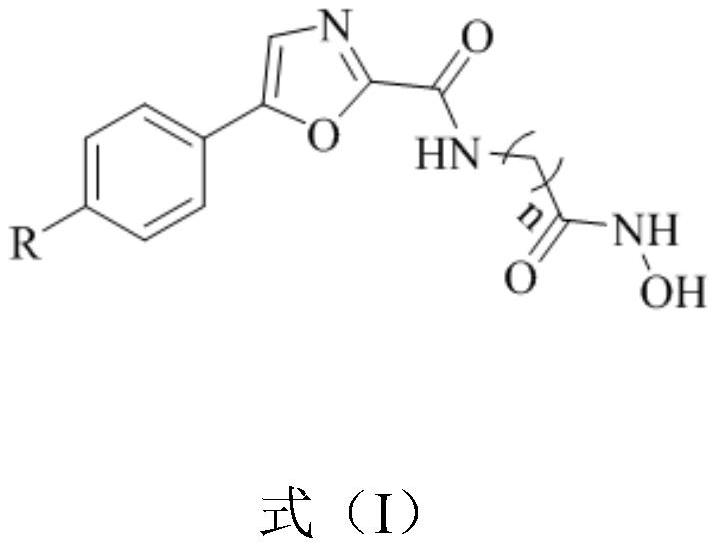
Oxazole histone deacetylase inhibitor and preparation method thereof
A deacetylase and inhibitor technology, applied in the field of new drug compounds, can solve the problems of unclear curative effect of B-cell lymphoma, poor curative effect and low activity of solid tumors, and achieve strong inhibitory effect, good effect and low overcoming activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
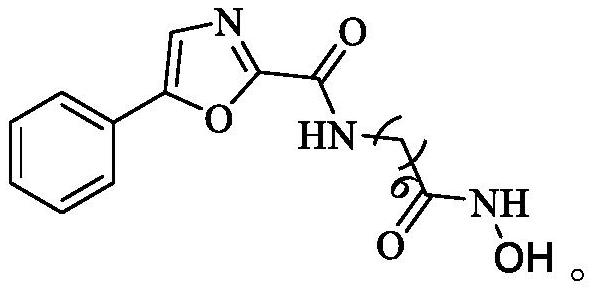
Embodiment 1
[0033] This embodiment provides a method for preparing an oxazole histone deacetylase inhibitor, comprising the following steps:
[0034] S1), respectively put 15mmol of hydroxylamine hydrochloride and 15mmol of KOH in a round bottom flask, add 25mL of anhydrous methanol to dissolve, stir in an ice bath for 30min and filter to obtain potassium hydroxylamine (NH 2 OK) solution, sealed for subsequent use;
[0035] S2), 1mmol of 7-(5-phenyloxazole-2-amide) heptanoic acid methyl ester was prepared in advance with 25mLNH 2 The OK solution was dissolved, and 20 mmol of hydroxylamine was added, and after stirring at room temperature for 1 h, the end point of the reaction was detected by TCL, and the solvent was removed under reduced pressure;
[0036] S3), add an appropriate amount of 2mol / L hydrochloric acid solution to acidify to PH=3-4, extract with dichloromethane (3×20mL), anhydrous MgSO 4 Dry and evaporate to dryness to obtain the crude product, denoted as 10d.
[0037] Pres...
Embodiment 2
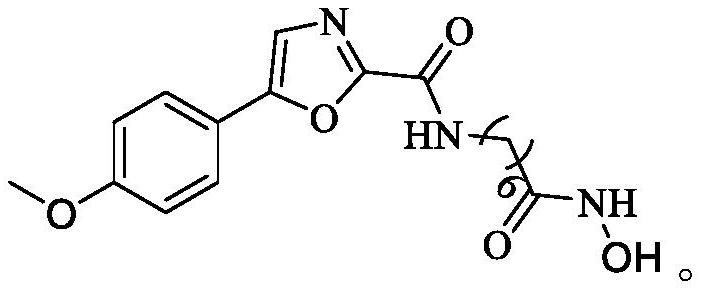
[0042] This embodiment provides a method for preparing an oxazole histone deacetylase inhibitor, comprising the following steps:
[0043] S1) Put 15mmol of hydroxylamine hydrochloride and 15mmol of KOH in a round-bottomed flask respectively, add 25mL of anhydrous methanol to dissolve, stir in an ice bath for 30min, and filter to obtain hydroxylamine potassium (NH 2 OK) solution, sealed for future use.
[0044] S2), 1mmol of 7-(5-(4-methoxyphenyloxazole)-2-amide) methyl heptanoate was prepared in advance with 25mLNH 2 The OK solution was dissolved, and 20 mmol of hydroxylamine was added, and after stirring at room temperature for 1 h, the end point of the reaction was detected by TCL, and the solvent was removed under reduced pressure;
[0045] S3), add an appropriate amount of 2mol / L hydrochloric acid solution to acidify to PH=3-4, extract with dichloromethane (3×20mL), anhydrous MgSO 4 Dry and evaporate to dryness to obtain the crude product, recorded as 9g.
[0046] Prese...
Embodiment 3
[0051] This embodiment provides a method for preparing an oxazole histone deacetylase inhibitor, comprising the following steps:
[0052] S1), respectively put 15mmol of hydroxylamine hydrochloride and 15mmol of KOH in a round bottom flask, add 25mL of anhydrous methanol to dissolve, stir in an ice bath for 30min and filter to obtain potassium hydroxylamine (NH2 OK) solution, sealed for future use.
[0053] S2), 1mmol of 7-(5-(4-chlorophenyloxazole)-2-amide) methyl heptanoate was prepared in advance with 25mLNH 2 The OK solution was dissolved, and 20 mmol of hydroxylamine was added, and after stirring at room temperature for 1 h, the end point of the reaction was detected by TCL, and the solvent was removed under reduced pressure;
[0054] S3), add an appropriate amount of 2mol / L hydrochloric acid solution to acidify to PH=3-4, extract with dichloromethane (3×20mL), anhydrous MgSO 4 Dry and evaporate to dryness to obtain the crude product, denoted as 10m.
[0055] Present em...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com