Crassostrea gigas DM9-domain-containing protein CgDM9CP-2, preparation method and application
A domain protein, long oyster technology, applied in the field of molecular biology, to achieve a strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
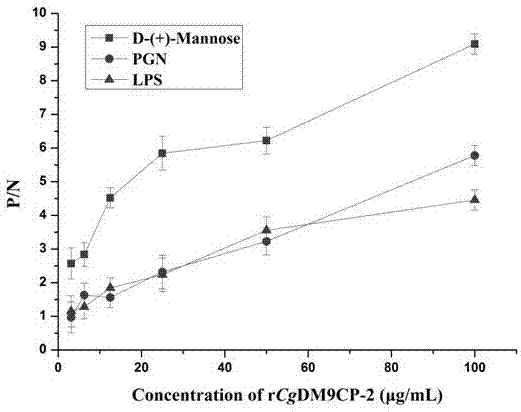
[0035] Experimental Example 1: Detection of the binding activity of the long oyster DM9 domain-containing protein CgDM9CP-2 of the present invention to Mannose, LPS, and PGN
[0036] Proceed as follows:
[0037] 1. Dissolve 10 μg of various PAMPs in 50 mM sodium carbonate-sodium bicarbonate buffer, 100 μl per well to coat the microtiter plate, 4°C, overnight.
[0038] 2. Wash with PBS-T 3 times, 5 min each time, add 200 μL 3% BSA to each well, and block at 37 °C for 1 h.
[0039] 3. Wash with PBS-T 3 times, 5 min each time, add 100 μL 2-fold serially diluted DM9 domain-containing protein CgDM9CP-2 of the present invention to each well, and incubate at 18°C for 3 h.
[0040] 4. Wash with PBS-T 3 times, 5 min each time, add 100 μL of diluted polyclonal antibody against the target protein (1:1000) to each well, and incubate at 37 °C for 1 h.
[0041] 5. Wash with PBS-T 3 times, 5 min each time, add 100 μL alkaline phosphatase-labeled goat anti-rat IgG (1:4000) to each well, a...
experiment example 2
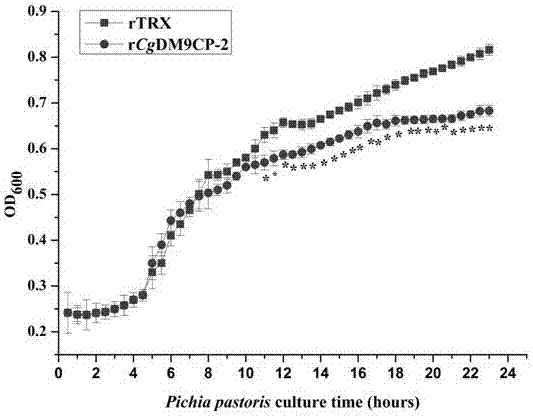
[0044] Experimental example 2: Antibacterial activity detection of the long oyster protein CgDM9CP-2 containing DM9 domain of the present invention
[0045] Specific steps are as follows:
[0046] 1. Culture and preparation of microorganisms
[0047] Pichia pastoris (a laboratory-preserved strain) was cultured in YPD medium at 28°C, 220 rpm, and when it reached the logarithmic growth phase, Tris-HCl (50 mmol L -1 , pH = 8.0) to dilute the bacteria so that the number of colonies per milliliter of bacteria liquid is about 1×10 3 CFU;
[0048] 2. Determination of antibacterial activity of recombinant protein CgDM9CP-2
[0049] Pichia pastoris in the logarithmic growth phase were collected by centrifugation and washed with TBS (50 mM Tris-Hcl, 150 mM NaCl) and resuspended (10 4 CFU). 50 μL of the DM9 domain-containing protein CgDM9CP-2 of the oyster of the present invention was incubated with an equal volume of Pichia pastoris for 2 h at room temperature. Take 20 μL of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com