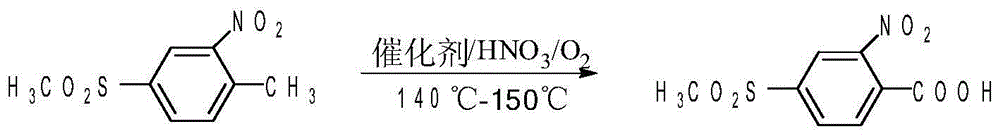
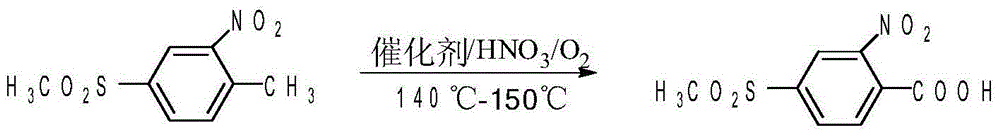
Method of preparing 2-nitro-4-methylsulfonyl benzoic acid
A technology of thiamphenicol benzoic acid and thiamphenicol toluene, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem that liquid-phase air oxidation is difficult to apply to industrial production, requires high equipment and technology, and is difficult Large-scale application and other issues, to achieve the effect of large industrial practical value, increase reaction yield, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 500mL reaction kettle equipped with a self-priming stirrer, add 140g 75% sulfuric acid, 20g 2-nitro-4-thiamphenicol toluene, 0.4g V 2 o 5 Stir and dissolve, close the system, connect the oxygen storage tank, adjust the stirring speed so that the gas holdup (volume) in the reaction solution reaches 15%, gradually heat up to 138-140°C, add 60g of 50% nitric acid to the system within 5-6 hours, and simultaneously Supplement oxygen to the system to keep the system pressure at 5×10 -3 MPa, react for 3 hours, and when the content of 2-nitro-4-thiamphenicol toluene is traced by liquid chromatography 98%. The filtrate was recovered and applied mechanically, 2-nitro-4 - The yield of thiamphenicol benzoic acid reaches 96.7% (calculated as 2-nitro-4-thiamphenicol toluene). 1g of product produces 6g of waste water, no waste gas and waste residue
Embodiment 2
[0029] 210g 75% sulfuric acid, 30g 2-nitro-4-thiamphenicol toluene, 0.3g V 2 o 5 Add it into a closed reaction kettle with a self-priming stirrer, stir and feed oxygen, adjust the temperature to 155°C, and adjust the stirring rate to make the gas holdup (volume) in the reaction solution reach 15%; add 90g of concentration dropwise 50% nitric acid, add it dropwise for 6 hours, add oxygen to the system as the reaction progresses, and keep the system pressure at 5×10 -3 MPa. The reaction speed is controlled by adjusting the nitric acid dropping rate and the gas-liquid ratio. After 2 hours of reaction after dropping, the liquid chromatography traces the content of 2-nitro-4-thiamphenicol toluene 98%. - The yield of 4-thiamphenicol benzoic acid was 88.7%.
Embodiment 3
[0031] 70g 75% sulfuric acid, 10g 2-nitro-4-thiamphenicol toluene, 1g V 2 o 5 Add it into a closed reaction kettle with a self-priming stirrer, stir and feed oxygen, adjust the temperature to 140°C, and adjust the stirring speed to make the gas holdup (volume) in the reaction solution reach 15%; add 30g concentration dropwise For 70% nitric acid, add dropwise for 7h. As the reaction proceeds, oxygen is added to the system to keep the system pressure at 5×10 -3 MPa. The reaction rate was controlled by adjusting the nitric acid drop rate and gas-liquid ratio. After 3 hours of reaction after the dropwise addition, when the content of 2-nitro-4-thiamphenicol toluene is traced by liquid chromatography 98%, and the filtered mother liquor is recovered and applied mechanically. - The yield of 4-thiamphenicol benzoic acid was 90.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com