A kind of preparation method of polilidomide
A technology of polidomide and amides, which is applied in the field of polidomide preparation, can solve the problems of low yield, large solvent consumption, low purity and the like, and achieves a high yield, low solvent consumption and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
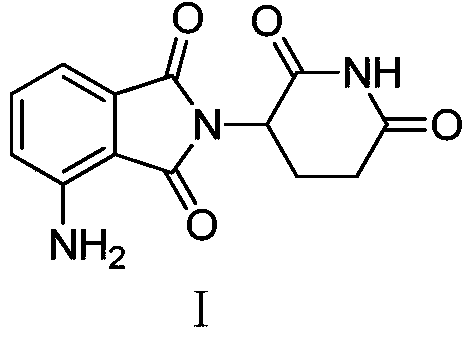
[0032] Preparation of Example 14-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione
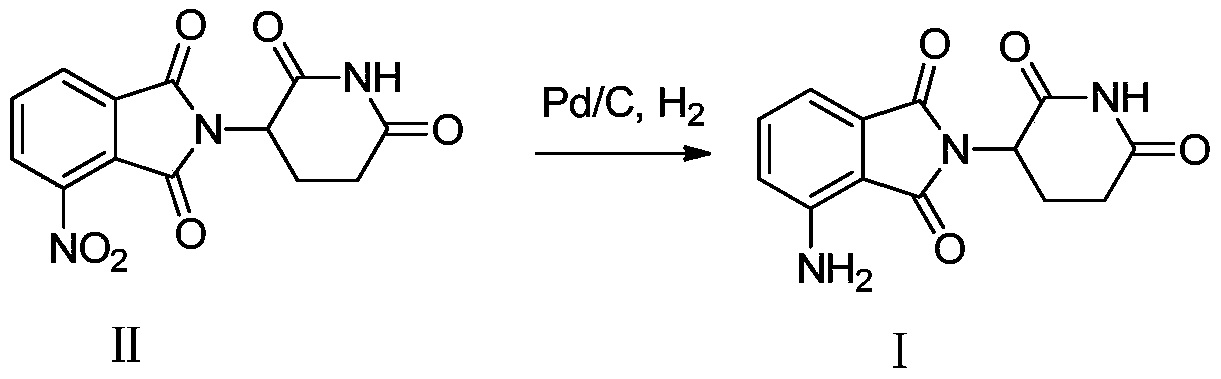
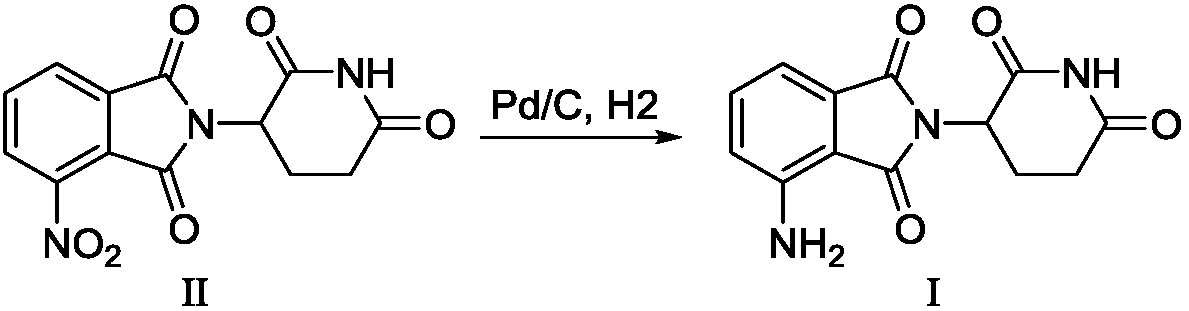
[0033] Mix 5 g of 1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-5-nitroisoindoline and 0.4 g of 10% palladium carbon into 50 mL of N,N - in dimethylacetamide, then carry out hydrogenation reaction at 30°C at 0.45Mpa for 7 hours, remove the catalyst by filtration, concentrate the filtrate under vacuum, and beat the concentrate with water overnight to obtain 3.6g of a yellow solid, the target compound. The yield is 80%, the purity measured by HPLC is 99.4%, and the melting point is 236-238°C.
[0034] 1 HNMR (DMSO-d 6 )δ:10.99(s,1H),7.42-7.47(m,1H),6.92-7.00(m,2H),6.52(s,2H),2.55-2.71(m,3H),1.88-2.05(m, 4H).
Embodiment 24
[0035] Preparation of Example 24-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione
[0036] Mix 5 g of 1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-5-nitroisoindoline and 0.4 g of 10% palladium carbon into 150 mL of N,N - in a mixed solvent of dimethylacetamide and ethanol (volume ratio 1:1), then carry out hydrogenation reaction at 50°C at 0.4Mpa for 7 hours, filter the catalyst, and concentrate the filtrate under vacuum, The concentrate was slurried with water overnight to obtain 3.4 g of a yellow solid, namely the target compound. The yield was 75%, and the purity measured by HPLC was 99%. NMR data is the same as in Example 1.
Embodiment 34
[0037] Preparation of Example 34-amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione
[0038] Mix 5 g of 1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-5-nitroisoindoline and 0.1 g of 10% palladium carbon into 105 mL of N,N -in a mixed solvent of dimethylacetamide and water (volume ratio 20:1), then hydrogenation reaction was carried out at 0.4Mpa at 40°C for 7 hours, the catalyst was filtered off, the filtrate was concentrated under vacuum, and the concentrated The mixture was slurried with water overnight to obtain 3.2 g of a yellow solid, namely the target compound. The yield was 71%, and the purity measured by HPLC was 99.2%. NMR data is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com