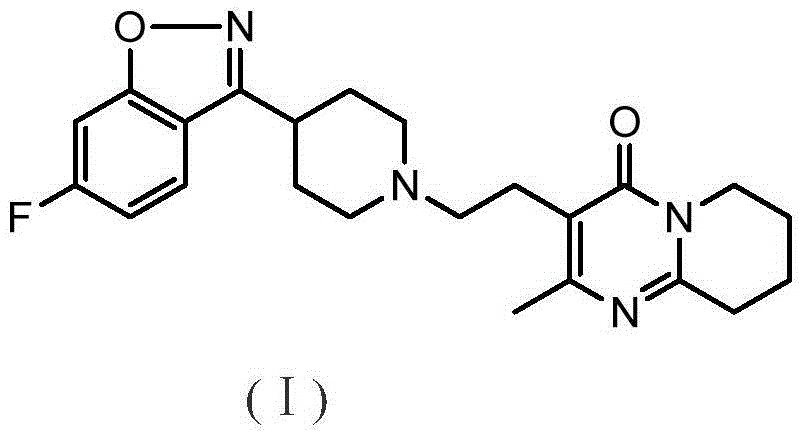
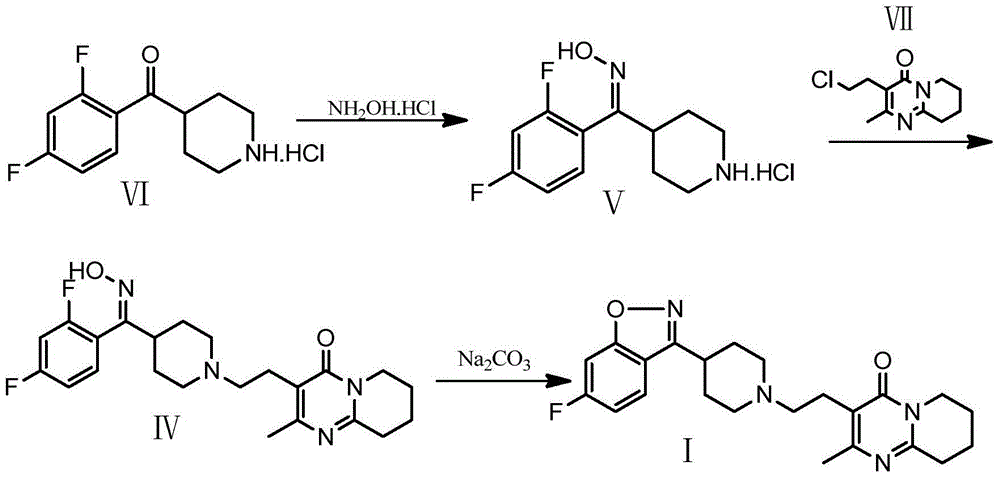
Method for preparing benzisoxazole antipsychotic drug risperidone
An antipsychotic drug, benzoisoxazole technology, applied in the field of preparation of risperidone, can solve the problems of high impurity control requirements, difficult industrialization, harsh conditions, etc., and achieve high product purity, low cost, and small environmental impact Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 32ml of 40wt% potassium hydroxide and 48ml of dichloromethane into a 250ml three-necked flask, and add (z)-[3-[4-[(2,4-difluorophenyl)methoximino]piperidinyl ]-1-ethyl]-2-methyl-6,7,8,9-4H-pyrido[1,2-a]pyrimidin-4-one 15g, stirred, heated to 38°C, heated to reflux for 2h, When the reaction system changed from turbid to clear, the reaction was continued for 1 hour, liquid separation, washing, crystallization, and drying were performed to obtain 12.30 g of risperidone. The purity of the obtained risperidone was 99.83%, the mono-impurity was 0.035%, and the yield was 86.03%.
Embodiment 2
[0029] Add 32ml of 35wt% potassium hydroxide and 32ml of dichloromethane into a 250ml three-necked flask, and add (z)-[3-[4-[(2,4-difluorophenyl)methoximino]piperidinyl ]-1-ethyl]-2-methyl-6,7,8,9-4H-pyrido[1,2-a]pyrimidin-4-one 15g, stirred, heated to 41°C, heated to reflux for 3h, When the reaction system changed from turbid to clear, the reaction was continued for 1 hour, liquid separation, washing, crystallization, and drying were performed to obtain 12.65 g of risperidone. The obtained risperidone had a purity of 99.88%, a monotonicity of 0.042%, and a yield of 88.45%.
Embodiment 3
[0031] Add 32ml of 40wt% sodium hydroxide and 64ml of dichloromethane into a 250ml three-necked flask, and add (z)-[3-[4-[(2,4-difluorophenyl)methoximino]piperidinyl ]-1-ethyl]-2-methyl-6,7,8,9-4H-pyrido[1,2-a]pyrimidin-4-one 15g, stirred, heated to 39°C, heated to reflux for 1.5h , when the reaction system changed from turbid to clear, the reaction was continued for 0.5h, liquid separation, washing, crystallization, and drying were performed to obtain 12.40 g of risperidone. The obtained risperidone had a purity of 99.84%, a mono-impurity of 0.041%, and a yield of 86.76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com