CDKN2A epitope peptide for detecting cervical cancer markers and application of CDKN2A epitope peptide
An antigenic epitope, cervical cancer technology, applied in animal/human peptides, tumor-specific antigens, tumor rejection antigen precursors, etc., can solve problems such as low sensitivity and poor specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, kit preparation
[0030] 1. Reagents: See Tables 1-6 for reagent preparation.
[0031] Table 1 antigen coating buffer (400ml volume)
[0032]
[0033] Table 2 Wash buffer (400ml volume)
[0034]
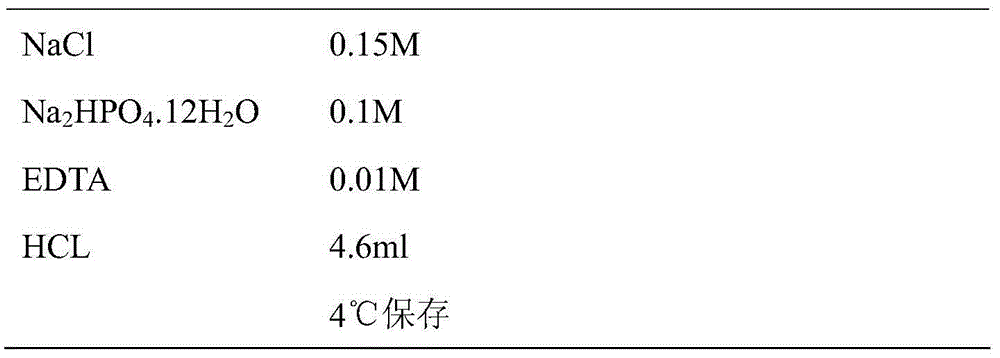
[0035] Table 3 Stop buffer (100ml volume)
[0036]
[0037] Table 4 working antigen
[0038]
[0039] Table 5 Secondary Antibody Standard Solution
[0040]
[0041] Table 6 Substrate Chromogenic Solution
[0042]
[0043] 2. Operation
[0044] (1) Coating: The microplate should be washed 3 times with washing buffer, the working antigen should be diluted to the working concentration with the coating solution, and coated on the microplate, overnight at 4°C.
[0045](2) Add glutamic acid: wash 3 times with washing buffer, dilute glutamic acid with coating solution to a concentration of 100 μg / ml, 200 μl per well, incubate at 37°C or room temperature for 1 hour;
[0046] (3) Add plasma and quality control control (primary antibody): wash the ...
Embodiment 2
[0050] Example 2, CDKN2A Auto IgG Antibody Detection of Cervical Patients
[0051] 1. Sample collection: 203 cases of malignant cervical tumors confirmed by radiological examination and histological examination were selected from the Second Hospital of Jilin University and Provincial Cancer Hospital. All serum samples had not undergone any anti-cancer treatment before collection, and had comprehensive clinical data and information. At the same time, 154 healthy control samples were recruited. Clinical interviews and imaging examinations ruled out the possibility of other diseases of the cervix. The healthy group and the cervical cancer group are comparable in gender and age (P>0.05)
[0052] 2. Test results: CDKN2A autoantibody expression level (see Table 7): There is a statistical difference between the cervical cancer group and the healthy control group (t=-4.480, P<0.001).
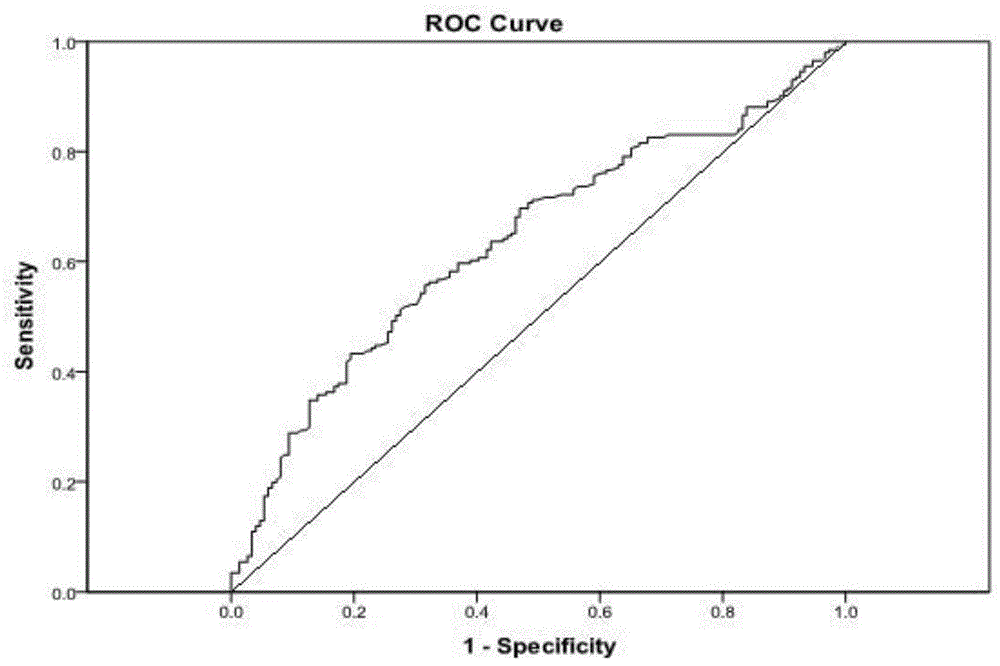
[0053] ROC curve analysis: the area under the curve of the cervical cancer group was 0.637 (SE=0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com