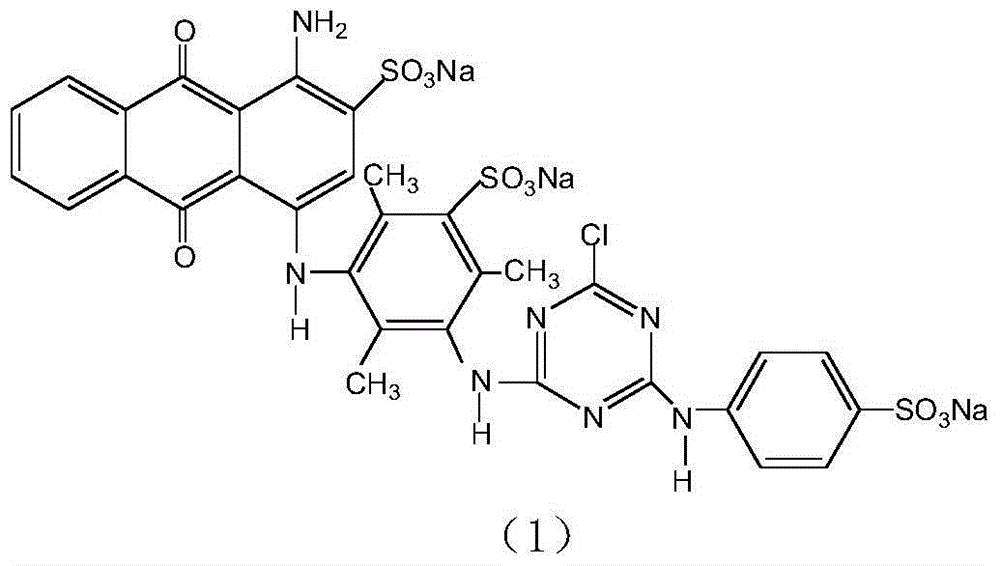
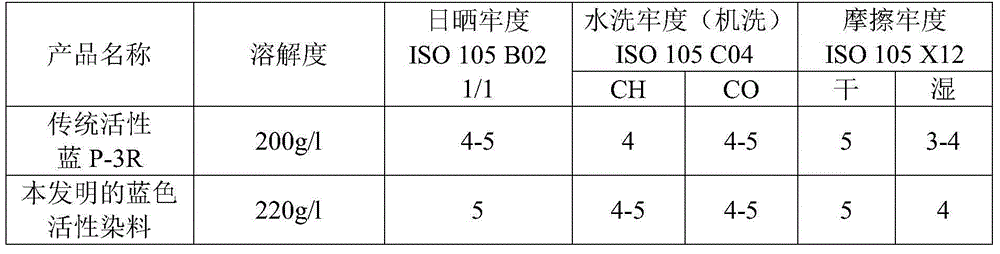
Method for preparing blue reactive dyes
A reactive dye, blue technology, applied in reactive dyes, azo dyes, organic dyes, etc., can solve the problems of insufficient wet rubbing fastness, small sewage discharge, and insufficiently bright color, achieving bright color, thorough reaction, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Primary condensation reaction: heat 100 parts of bromic acid to 80°C, disperse for 2 hours, add 102 parts of M acid solution, and maintain the temperature at 95°C for 12 hours in the presence of 20 parts of catalyst copper sulfate pentahydrate to obtain primary condensation For the end point of chromatographic detection, it is required that HPLC>95%;
[0033] 2) Refining: Cool down the primary condensate to 0-20°C, adjust the pH=1-2 with hydrochloric acid, and then add 8% sodium chloride in total volume to salt out and purify to obtain the refined product;
[0034] 3) Secondary condensation reaction: after dissolving the refined product, react with 90 parts of cyanuric chloride at pH=5.2-5.5, temperature 5-7°C for 4 hours to obtain a secondary condensation liquid;
[0035] 4) Tertiary condensation reaction: Add 90 parts of p-aminobenzenesulfonic acid powder into the secondary condensation liquid, react for 8 hours at a temperature of 45-48°C, pH=6-7, and perform TLC ...
Embodiment 2
[0038] 1) Primary condensation reaction: heat 100 parts of bromic acid to 80°C, disperse for 2 hours, add 104 parts of M acid solution, and maintain the temperature at 93°C for 10 hours in the presence of 22 parts of catalyst ferrous chloride mixture to obtain a Condensate, as the end point of chromatographic detection, requires HPLC>95%;
[0039] 2) Refining: cool down the primary condensate to 0-20°C, adjust the pH=1-2 with hydrochloric acid, and then add 10% of the total volume of sodium chloride to salt out and purify to obtain the refined product;
[0040] 3) Secondary condensation reaction: after dissolving the refined product, react with 92 parts of cyanuric chloride at pH=5.2-5.5, temperature 5-7°C for 6 hours to obtain a secondary condensation liquid;
[0041] 4) Tertiary condensation reaction: Add 93 parts of p-aminobenzenesulfonic acid powder into the secondary condensation liquid, react for 8 hours at a temperature of 45-48°C, pH=6-7, and perform TLC chromatography...
Embodiment 3
[0044] 1) Primary condensation reaction: heat up 100 parts of bromine to 80°C, disperse for 2 hours, add 106 parts of M acid solution, and add 24 parts of the catalyst mixture of copper sulfate pentahydrate and ferrous chloride (the ratio of the two is 1:1) In the case of existence, maintain the temperature at 90°C for 12 hours to obtain a condensate, and do the chromatographic detection end point, requiring HPLC>95%;
[0045] 2) Refining: cool down the primary condensate to 0-20°C, adjust the pH=1-2 with hydrochloric acid, and then add 10% of the total volume of sodium chloride to salt out and purify to obtain the refined product;
[0046] 3) Secondary condensation reaction: dissolve the refined product and react with 93 parts of cyanuric chloride at pH=5.2-5.5, temperature 5-7°C for 4 hours to obtain a secondary condensation liquid;
[0047] 4) Tertiary condensation reaction: Add 93 parts of p-aminobenzenesulfonic acid powder into the secondary condensation liquid, react for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com