A kind of synthetic method of isotope-labeled metilcarb-d3
A technology of isotope labeling and synthesis method, which is applied in the field of preparation of organic compounds, can solve problems such as difficult introduction of deuterated isotope labels, and achieve high selectivity, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
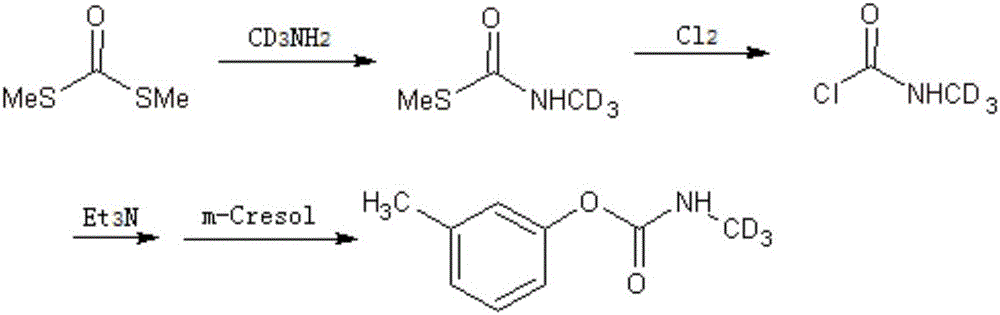
[0017] Isotope-labeled Methiocarb-D 3 The synthetic method of monomethylamine hydrochloride-D 3 The aqueous solution of the dithiocarboxylate solution is added dropwise to the dimethyl dithiocarboxylate solution, and the intermediate S-methyl-N-methylcarboxylate is obtained after the reaction, and the above-mentioned intermediate is slowly passed into Cl 2 , adding acid-binding agent and m-cresol successively to prepare the marker Medicarb-D 3 , take the following steps:
[0018] (1) in N 2 Under protection, monomethylamine hydrochloride-D 3 Dissolve in water, add dropwise into the dimethyl dithiocarboxylate solution within 5-10 minutes, use alkali to keep the pH of the reaction solution = 8-9, the reaction is mild and exothermic, keep the reaction temperature at 30-60°C, and react for 4-8 hours , until the raw material disappears, and after extraction with dichloromethane, the organic phases are combined, and anhydrous Na 2 SO 4 Dry overnight and concentrate to give int...
Embodiment 1
[0028] in N 2 Under protection, 15mmol monomethylamine hydrochloride-D 3 Dissolve in a small amount of water, add dropwise to 50 mmol dimethyl dithiocarboxylate solution within 5-10 min, and keep the pH of the reaction solution at 8-9 with NaOH. The reaction was mildly exothermic, and the reaction was maintained at 30°C for 8 hours until the raw materials disappeared and the reaction ended. Extract with dichloromethane, combine the organic phases, anhydrous Na 2 SO 4Dry overnight and concentrate to give intermediate S-methyl-N-methylcarboxylate-D as a colorless oil 3 .
[0029] The intermediate S-methyl-N-methylcarboxylate-D 3 Dissolve in dimethyl dithiocarboxylate, cool to -30°C, slowly pass in Cl while stirring 2 , the mixture turned pale yellow during the process, and gradually faded until the color of the reaction solution was colorless, and the reaction was stirred for 1 h.
[0030] in N 2 Under protection, triethylamine was added to the reaction solution to keep ...
Embodiment 2
[0032] in N 2 Under protection, 15mmol monomethylamine hydrochloride-D 3 Dissolve with a small amount of water, add dropwise to 15 mmol dimethyl dithiocarboxylate solution within 5 to 10 min, and use Na 2 CO 3 Keep the pH of the reaction solution = 8-9. The reaction was mildly exothermic, and the reaction was maintained at 60°C for 6 hours until the raw materials disappeared, and the reaction ended. Extract with dichloromethane, combine the organic phases, anhydrous Na 2 SO 4 Dry overnight and concentrate to give intermediate S-methyl-N-methylcarboxylate-D as a colorless oil 3 .
[0033] The intermediate S-methyl-N-methylcarboxylate-D 3 Dissolve in dimethyl dithiocarboxylate, cool to -20°C, slowly pass in Cl while stirring 2 , the mixture turned pale yellow during the process, gradually faded until the color of the reaction solution was colorless, and stirred for 4h.
[0034] in N 2 Under protection, DIEA was added to the reaction solution to keep the pH of the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com