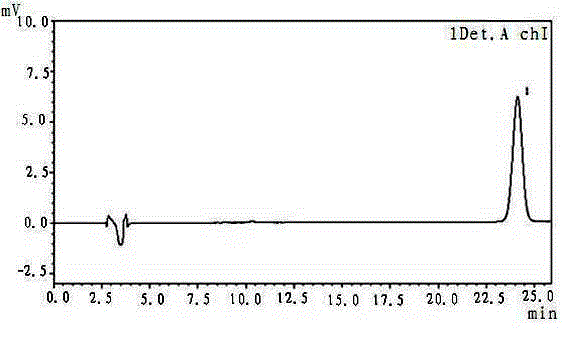
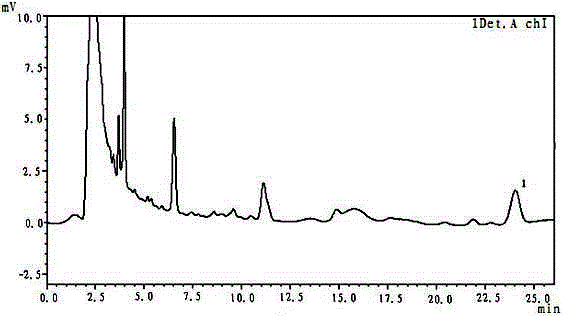
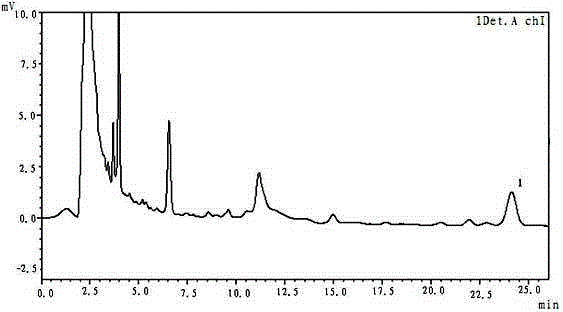
Method for determining content of ferulic acid in pill
A ferulic acid and content technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as low quality, unstable drug quality, and no regulations on quality testing methods, and achieve stable and high quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The method for determining the content of ferulic acid in pills, the instruments and reagents used: liquid chromatograph model: LC-20AD; chromatographic column: 4.6mm×250mm; ferulic acid reference substance: provided by the National Institute for the Control of Pharmaceutical and Biological Products ; Methanol is chromatographically pure, acetic acid is chromatographically pure;
[0020] The method includes the following specific steps:
[0021] A. Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler, methanol and 1% acetic acid are used as mobile phase at a volume ratio of 30:70, detection wavelength is 320nm, column temperature is room temperature, theoretically The number calculated based on the ferulic acid peak should not be less than 5000;
[0022] B. Preparation of reference substance solution: Take an appropriate amount of ferulic acid reference substance, accurately weigh it, and add methanol to make a solution...
Embodiment 2
[0026] The method for determining the content of ferulic acid in pills, the instruments and reagents used: liquid chromatograph model: LC-20AD; chromatographic column: 4.6mm×250mm; ferulic acid reference substance: provided by the National Institute for the Control of Pharmaceutical and Biological Products ; Methanol is chromatographically pure, acetic acid is chromatographically pure;
[0027] The method includes the following specific steps:
[0028] A. Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler, methanol and 1% acetic acid are used as mobile phase at a volume ratio of 30:70, detection wavelength is 320nm, column temperature is room temperature, theoretically The number calculated based on the ferulic acid peak should not be less than 5000;
[0029] B. Preparation of reference substance solution: Take an appropriate amount of ferulic acid reference substance, accurately weigh it, and add methanol to make a solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com