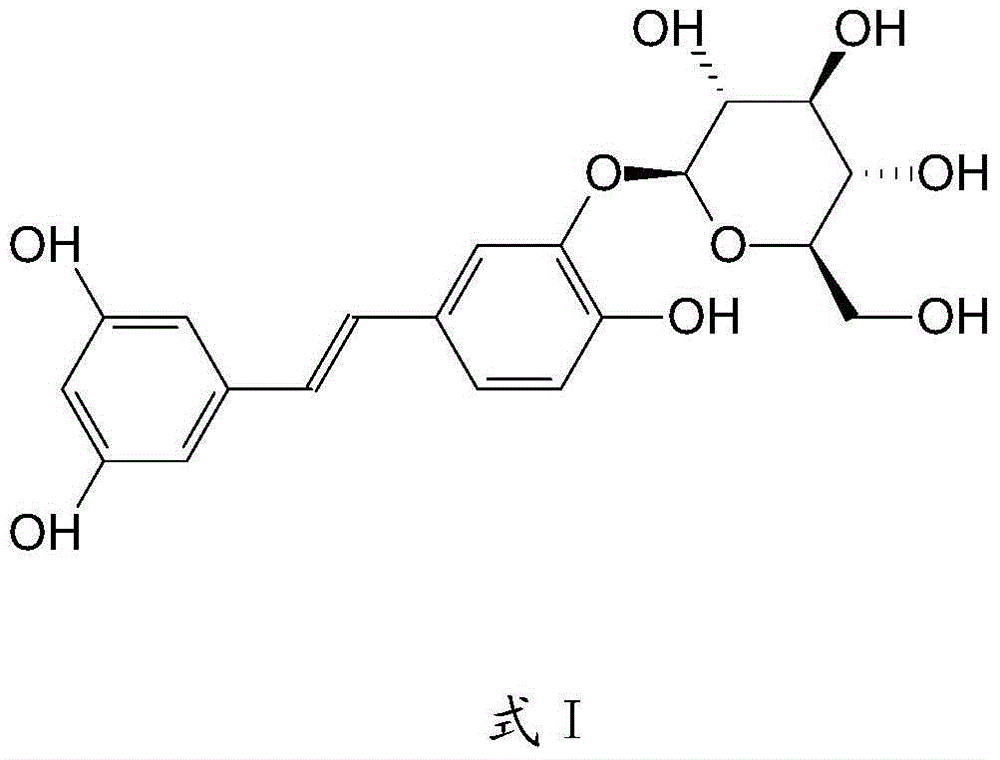
Application of 3,5,3',4'-trihydroxy-stilbene-3'-b-D-glucoside in preparation of medicines for treating cancers
A technology of trizaperoside and drugs, which is applied in the field of application of trizaperoside in the preparation of drugs for treating cancer, and can solve problems such as cancer recurrence and drug resistance of cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
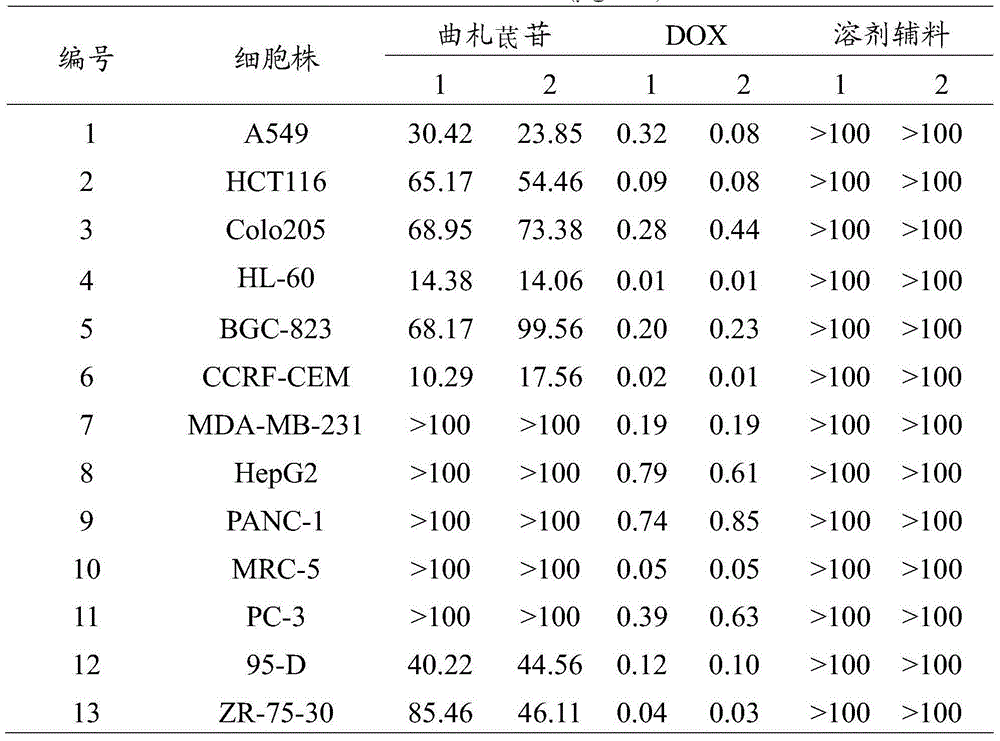
[0040] Embodiment 1MTT method detects the cytotoxic effect of trizapereside on human tumor cells cultured in vitro and human normal cells
[0041] Take A549, HCT116, colo205, HL-60, BGC-823, CCRF-CEM, MDA-MB-231, HepG2, 95-D, ZR-75-30, PANC-1, PC-3 in the logarithmic growth phase and MRC-5 cells, the adherent cells were first digested and then prepared with fresh culture medium to make 4-5×10 4 per mL, add 100 μL to each well of a 96-well plate, and place at 37°C, 5% CO 2 Cultured in an incubator. After 24 hours, the samples were added, and the test sample tristilbene, the positive control doxorubicin (DOX) and the solvent excipient hydroxypropyl-β-cyclodextrin were diluted with PBS(-) to a final concentration of 100, 10, 1, 0.1, 0.01, 0.001, 0.0001 μg / mL, diluted and added to each well (10 μL / well), set 3 replicate wells for the same sample and the same concentration. After continuing to culture for 72 hours, add 20 μL of 5 mg / mL MTT solution to each well, place in the inc...
Embodiment 2
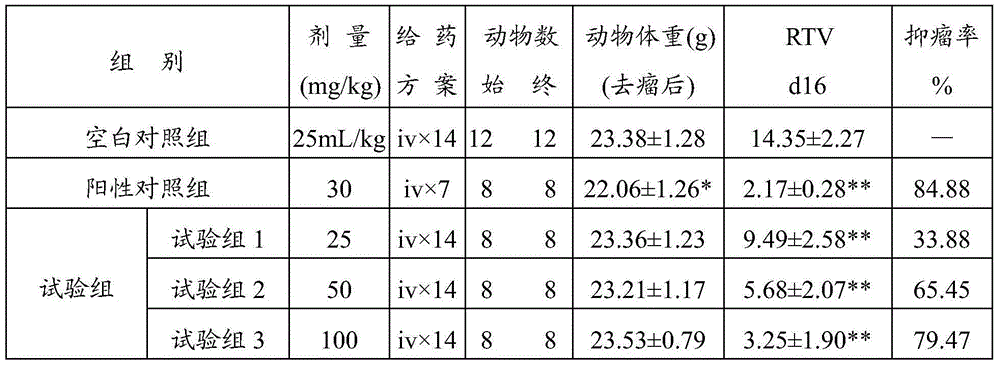
[0049] Example 2 Curative effect of tristilbene on nude mouse xenograft model of human tumor
[0050] The well-grown A549 solid tumor was cut into uniform small pieces about 3 mm in size under aseptic conditions, and a piece was inoculated subcutaneously in the right armpit of each nude mouse with a trocar. Thirteen days after inoculation, the tumors were regrouped according to the size of the tumors, and the nude mice with too large and too small tumors were eliminated. The average tumor volume of each group was basically the same, and the following 5 groups were set up:
[0051] Blank control group: sterile normal saline (0.9% sodium chloride injection); (25mL / kg, iv×14); 12 nude mice;
[0052] Positive control group: CTX (30mg / kg, iv×7); diluted with sterile saline; 8 nude mice;
[0053] Test group 1: Trizastilbene L (25mg / kg, iv×14); diluted with PBS(-); 8 nude mice;
[0054] Test group 2: Tristilbene M (50mg / kg, iv×14); diluted with PBS(-); 8 nude mice;
[0055] Test g...
Embodiment 3
[0080] The preparation of embodiment 3 medicines
[0081] Weigh the following raw materials:
[0082]
[0083]
[0084] The above raw materials are pulverized, mixed uniformly, granulated, dried, and compressed into tablets to obtain trizaperidin tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com