Process for forming an aramid copolymer
A technology of polymer and polymer solution, applied in the field of preparing aromatic polyamide polymer, can solve the problem of not allowing the preparation of aromatic polyamide polymer and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0057] NMP, CaCl 2 , DAPBI, PPD and TCl were obtained from commercial sources.
example 1
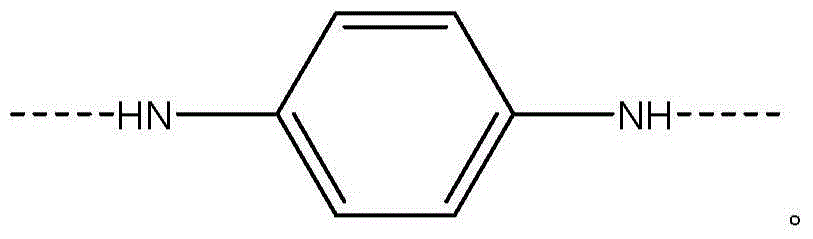
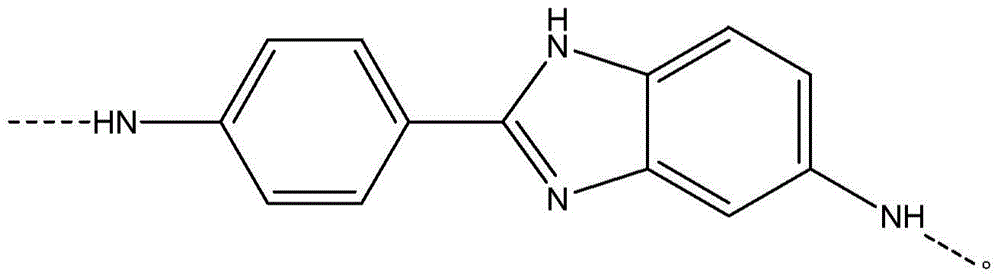
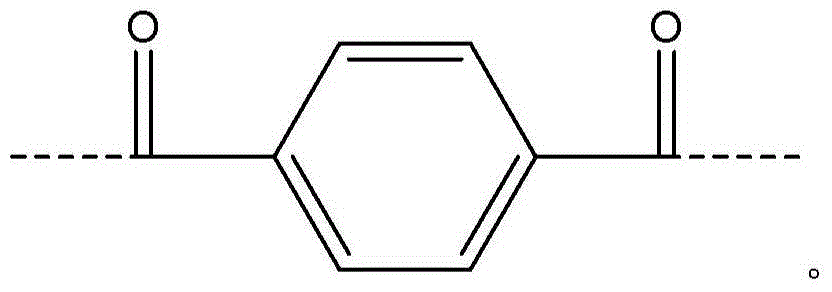
[0059] Add 5.38% CaCl to the FM130D Littleford reactor 2 31.82 kg of NMP solvent ("c" value 5.38). Then 2180 g of DAPBI ("b" value 70) was added. The contents were then cooled to 10°C. 707 g of terephthaloyl chloride was added to form a prepolymer solution. The prepolymer solution was then cooled to 10°C, and 455 g of PPD ("y" value 30) was dissolved in the prepolymer mixture. Then 2122 g of terephthaloyl dichloride were added. The product of (b×c) of the polymer is equal to 377.
[0060] The reaction had 12% solids on a polymer basis and 14.7% solids on a total monomer basis. The final inherent viscosity was 6.5 dl / g.
example 2
[0068] Example 1 was repeated, however adding terephthaloyl dichloride to DAPBI in three additions. After each addition, the mixture was cooled to 10 °C. The result was a polymer solution and polymer similar to Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inherent viscosity | aaaaa | aaaaa |
| Inherent viscosity | aaaaa | aaaaa |
| Inherent viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com