Preparation and modification methods for solid material Zr-CN for adsorbing CO2
A solid material and modification technology, applied in separation methods, chemical instruments and methods, and other chemical processes, can solve the problems of small adsorption capacity, high adsorption-desorption temperature, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0024] a. Dissolve 8mmol potassium ferrocyanide in 80ml deionized water to form solution A1;
[0025] b. Add 4 mmol of zirconium oxychloride octahydrate to the A1 solution, and stir at 30° C. for 3 h to obtain the first solid product;
[0026] c. washing the obtained first solid product with deionized water until the solution is neutral;
[0027] d. The washed first solid product was dried at 30° C. for 12 hours, and then dried in a vacuum oven at 80° C. for 6 hours to obtain the solid material Zr-CN.
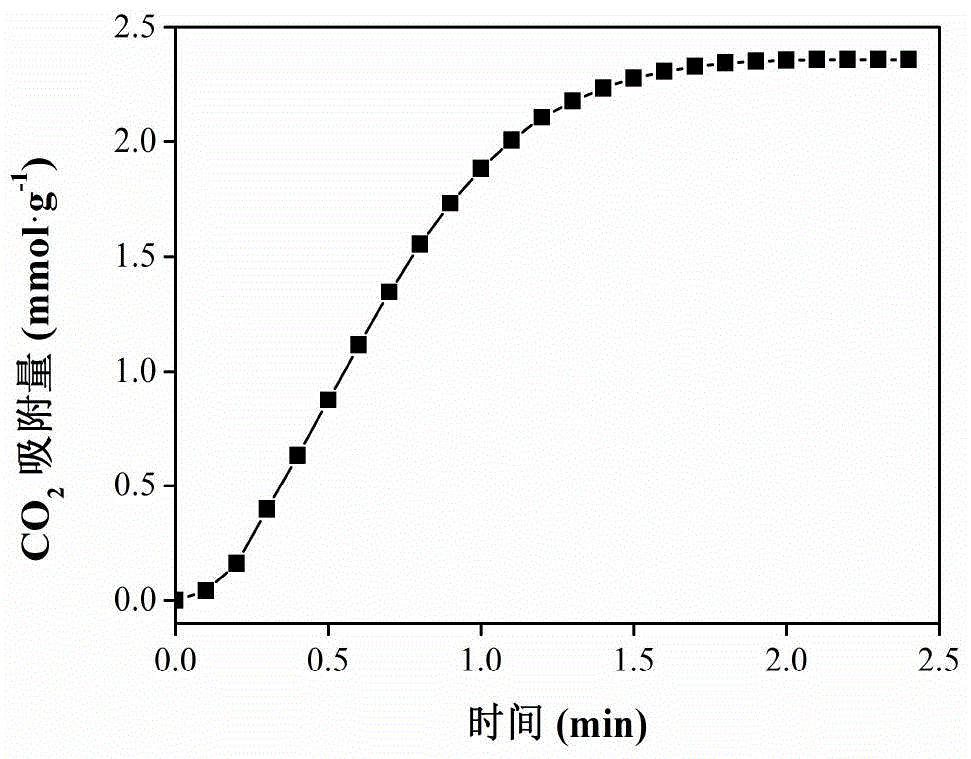
[0028] According to the obtained solid material Zr-CN of embodiment 1 to CO 2 The curve of the adsorption capacity with time is shown in the appendix figure 1 As shown, it can be seen from the figure that the Zr-CN to CO 2 The adsorption increases with time, and the maximum adsorption capacity is 2.35mmol·g -1 .
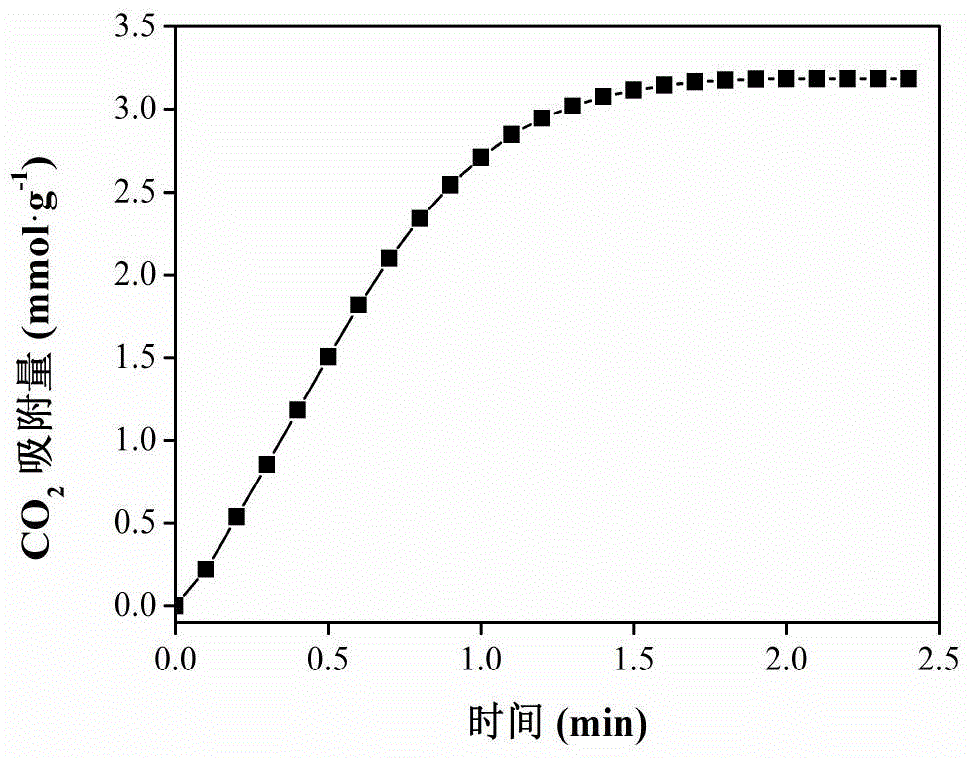
preparation example 2
[0030] a. Dissolve 8mmol potassium ferrocyanide in 80ml deionized water to form solution A1;
[0031] b. Add 8 mmol of zirconium oxychloride octahydrate to the A1 solution, and stir at 30°C for 4 hours to obtain the first solid product;
[0032] c. washing the obtained first solid product with deionized water until the solution is neutral;
[0033] d. The washed first solid product was dried at 40° C. for 18 hours, and then dried in a vacuum oven at 80° C. for 7 hours to obtain the solid material Zr-CN.
preparation example 3
[0035] a. Dissolve 8mmol potassium ferrocyanide in 80ml deionized water to form solution A1;
[0036] b. Add 12 mmol of zirconium oxychloride octahydrate to the A1 solution, and stir at 30° C. for 5 h to obtain the first solid product;
[0037] c. washing the obtained first solid product with deionized water until the solution is neutral;
[0038] d. The washed first solid product was dried at 50° C. for 24 hours, and then dried in a vacuum oven at 80° C. for 8 hours to obtain the solid material Zr-CN.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com