Formulations involving solvent/detergent-treated plasma (s/d plasma) and uses thereof
A detergent, plasma technology, applied in the field of treatment of diseases such as musculoskeletal diseases, can solve the problems of inability to provide scaffolds, variable bacteria, ineffective non-lipid enveloped viruses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1: Comparison between Human Solvent / Detergent Treated Plasma (S / D Plasma) and Human Fresh Frozen Plasma (FFP)
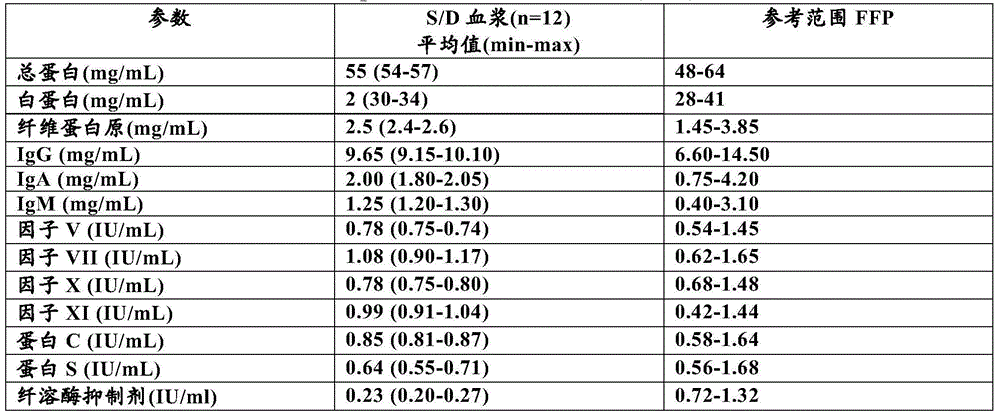
[0157] Compositional Characteristics of Exemplary Formulations
[0158] S / D plasma (i.e. obtained from Octapharma AG (Lachen, Switzerland) ) provides a comparison between the compositional characteristics of S / D plasma and human fresh frozen plasma (FFP), as shown in Table 1. Based on this information, the products have largely comparable plasma protein compositions; S / D plasma may show significantly lower levels of plasmin inhibitors.
[0159] Table 1: Based on S / D plasma of product topic material (specifically ) and the composition characteristics of fresh frozen plasma (FFP)
[0160]
[0161] Comments on S / D plasma (specifically ) and the composition of fresh frozen plasma, Svae et al. concluded in 2007 that the manufacturing method (including S / D treatment) did not cause a critical decrease in the activity of coagulation factors and nat...
Embodiment 2
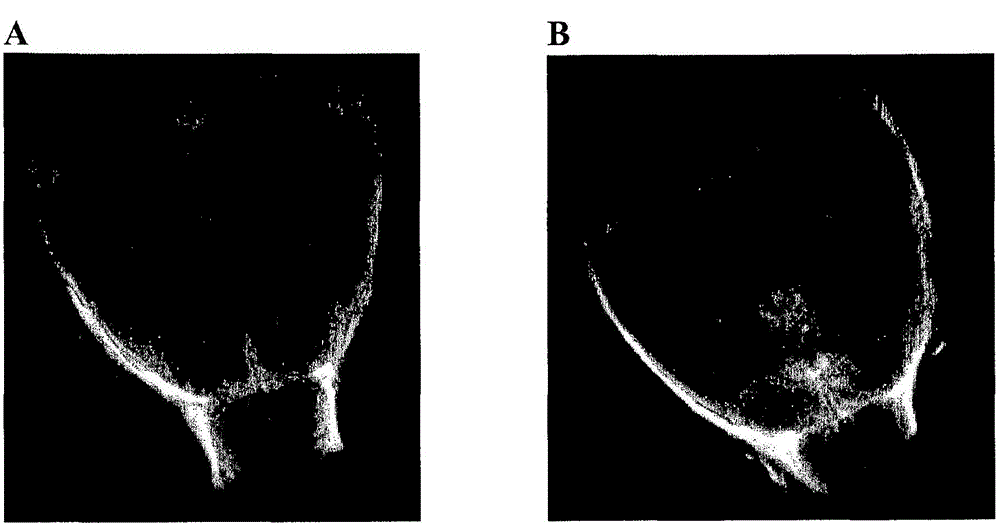
[0178] Example 2: Human solvent / detergent (S / D) treated plasma ( ) and hyaluronic acid (HA) for gel formation
[0179] clot / gel formation with HA
[0180] Human S / D plasma (specifically ) performance. The synovial fluid is brought into contact with a formulation according to one embodiment of the invention in a ratio of 1:1 (v / v), said formulation comprising Hyaluronic acid (10mg / ml sodium hyaluronate, 1.8-2.10 6 Molecular weight of Da, provided by Contipro, Czech Republic) and CaCl 2 . Tried three kinds of CaCl 2 Concentrations, i.e. 0, 2 and 4mg / ml CaCl 2 . Gel formation was assessed visually after different time points (20-30 min, 1 h, 2 h) using a timer. Viscosity was evaluated by measuring the time required for the solution to reach a defined mark on the vessel wall when the reaction tube was turned upside down. The test was performed twice on synovial fluid from 2 arthritic patients.
[0181] Incubate with synovial fluid containing 4mg / ml CaCl 2 The prep...
Embodiment 3
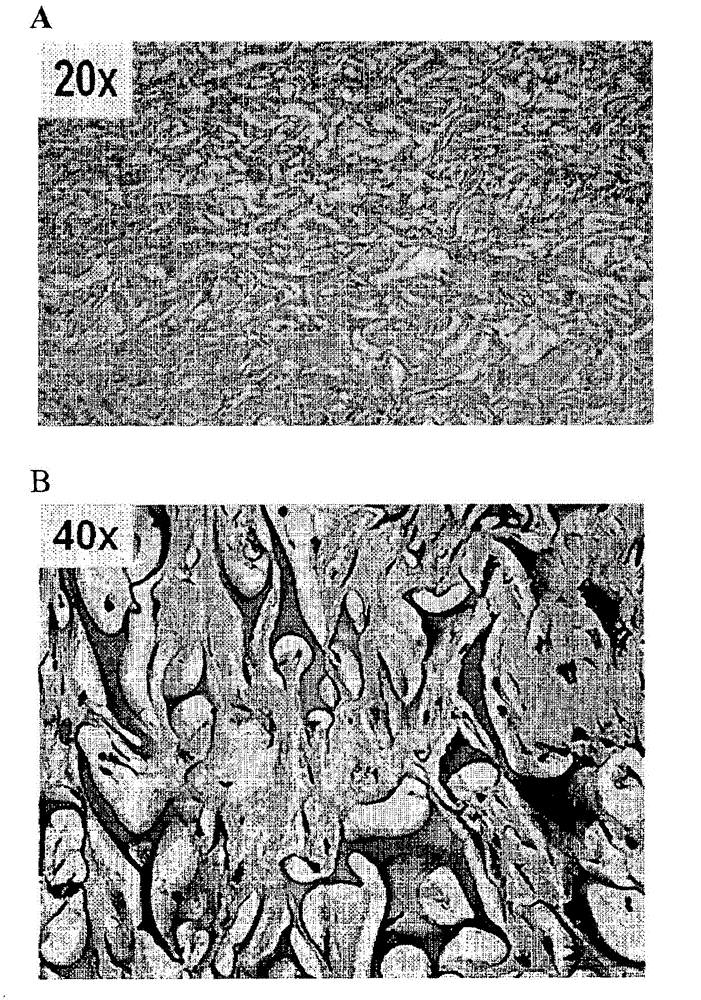
[0205] Example 3: Human S / D plasma (such as ) and the gel formation of the cell composition
[0206] and contain Ca 2+ Clot / Gel Formation of Cell Culture Media
[0207] For several cell-free, but containing human S / D plasma (specifically ), conventional medium (containing CaCl 2 ) and human serum preparations were tested for clot formation. Different conditions were tested to verify the Clot formation at 37°C under the following conditions: different concentrations, for example, 5%, (v / v) 7.5% v / v or 10% v / v; different conventional media, for example, DMEM, MEM, PBS or PBS+CaCl 2 and the absence or presence of 5 or 10% (v / v) serum. When combined in the absence of cells Regular medium (which contains CaCl 2 ) and serum, clot formation was visually observed.
[0208] It can be concluded that the presence of serum as well as calcium in the medium appears to be necessary for clot formation in the absence of cells.
[0209] and clot / gel formation of cellular compo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Growth factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com