Vaccine preparation as well as production method and use of vaccine preparation
一种制剂、疫苗的技术,应用在生物化学设备和方法、医药配方、非有效成分的医用配制品等方向,能够解决装载效率有待提高等问题,达到实现免疫激活效果、强烈免疫激活效果、提升免疫激活能力的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] In this embodiment, the vaccine vector is prepared by the following method, and the specific steps are as follows:
[0061] Disperse 5 g of the artificially cultivated Lactobacillus casei sludge in 100 mL of 0.01 mol / L hydrochloric acid solution to obtain a microbial suspension, and transfer it to a hydrothermal reaction kettle placed in a constant temperature box to maintain a pressure of 3 MPa at 180 ° C. Heating at a constant temperature for 10 hours, washing the obtained precipitate with pure water and freeze-drying to obtain the vaccine carrier (DB).
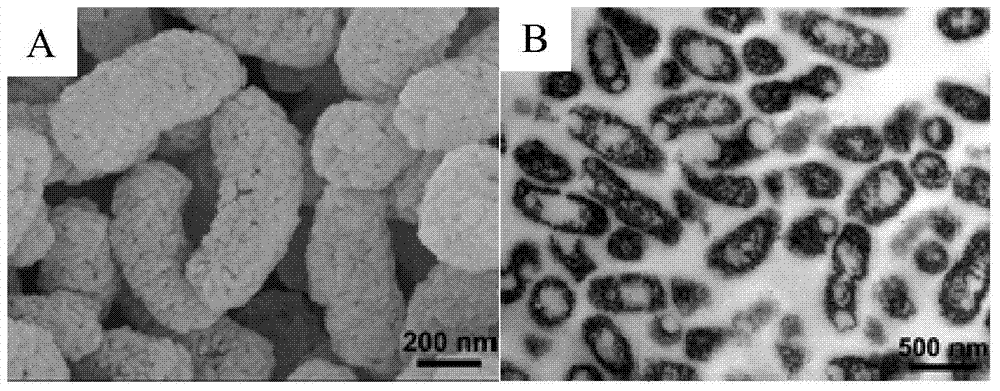
[0062] Utilize scanning electron microscope (JEOL, JSM-6700F) and transmission electron microscope (JEOL, JEM-1400) to carry out the characterization of the prepared vaccine carrier, as figure 1 As shown, the prepared vaccine vector retained the morphology characteristics of bacilli. In addition, a porous microstructure with an average pore diameter of 27.66 nm was formed on the surface of the carrier by virtue of t...
Embodiment 2
[0064] In this embodiment, the vaccine vector is prepared by the following method, and the specific steps are as follows:
[0065] Disperse 2 g of the artificially cultivated Lactobacillus casei sludge in 100 mL of 0.1 mol / L hydrochloric acid solution to obtain a microbial suspension, and transfer it to a hydrothermal reaction kettle placed in a constant temperature box to maintain a pressure of 2 MPa at 200 ° C. Heating at a constant temperature for 12 hours, washing the obtained precipitate with pure water and freeze-drying to obtain the vaccine carrier.
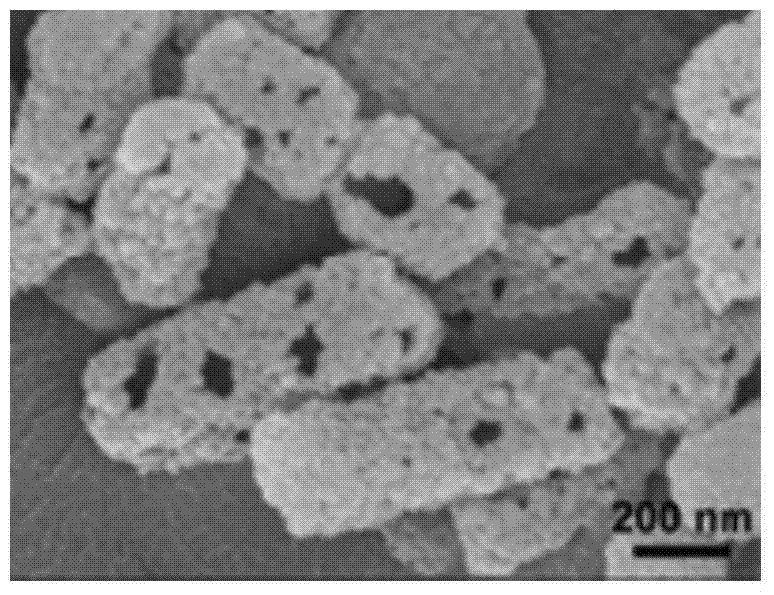
[0066] The prepared vaccine carrier was characterized by scanning electron microscopy, as shown in figure 2 As shown, due to the hydrothermal reaction in the acidic solvent environment, a large number of macroporous structures are formed on the surface of the carrier material.
Embodiment 3
[0068] In this embodiment, the vaccine vector is prepared by the following method, and the specific steps are as follows:
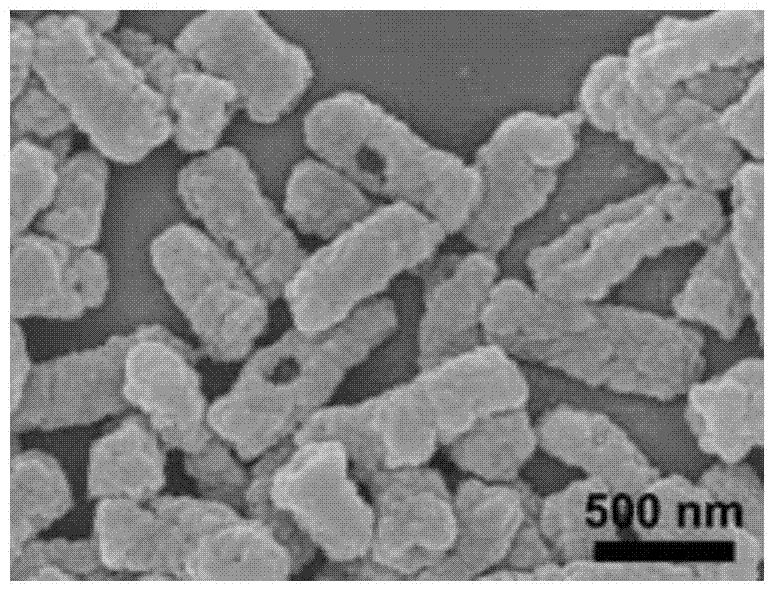
[0069] Disperse 5 g of the artificially cultivated Lactobacillus casei sludge in 100 mL of 0.01 mol / L hydrochloric acid solution to obtain a microbial suspension, and transfer it to a hydrothermal reaction kettle placed in a constant temperature box to maintain a pressure of 3 MPa at 100 ° C. Heating at a constant temperature for 72 hours, washing the obtained precipitate with pure water and freeze-drying to obtain the vaccine carrier.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com