Preparation method and application of a water-stable nanocomplex
A complex and water-stabilized technology, which is applied in the field of material science, can solve the problems of poor water stability of complexes, limit large-scale applications, and low skeleton density, and achieve the effects of long reaction cycle, simple method, and high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of triazine-based complex crystals in the prior art:
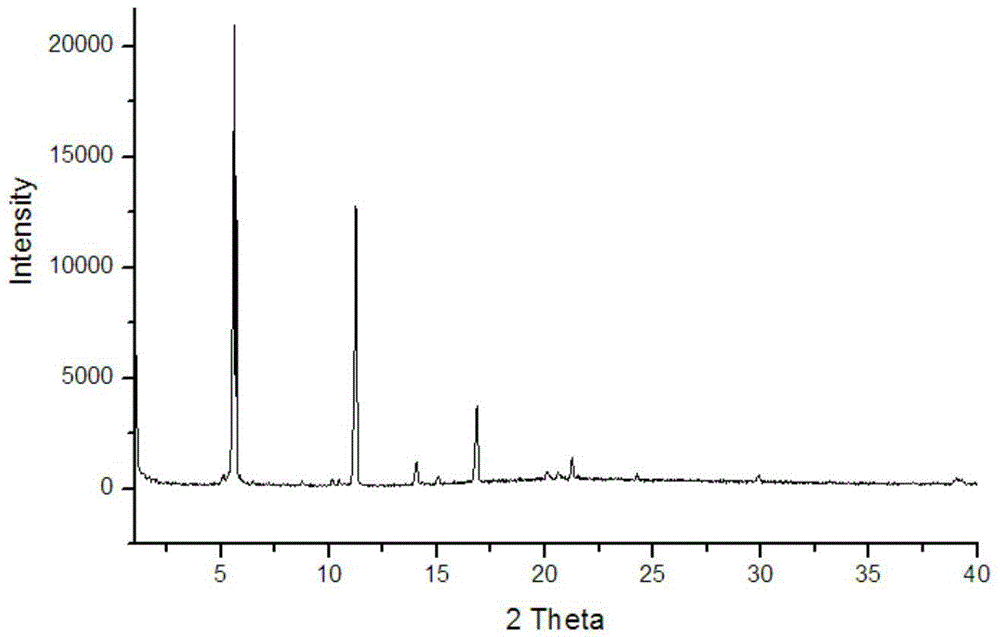
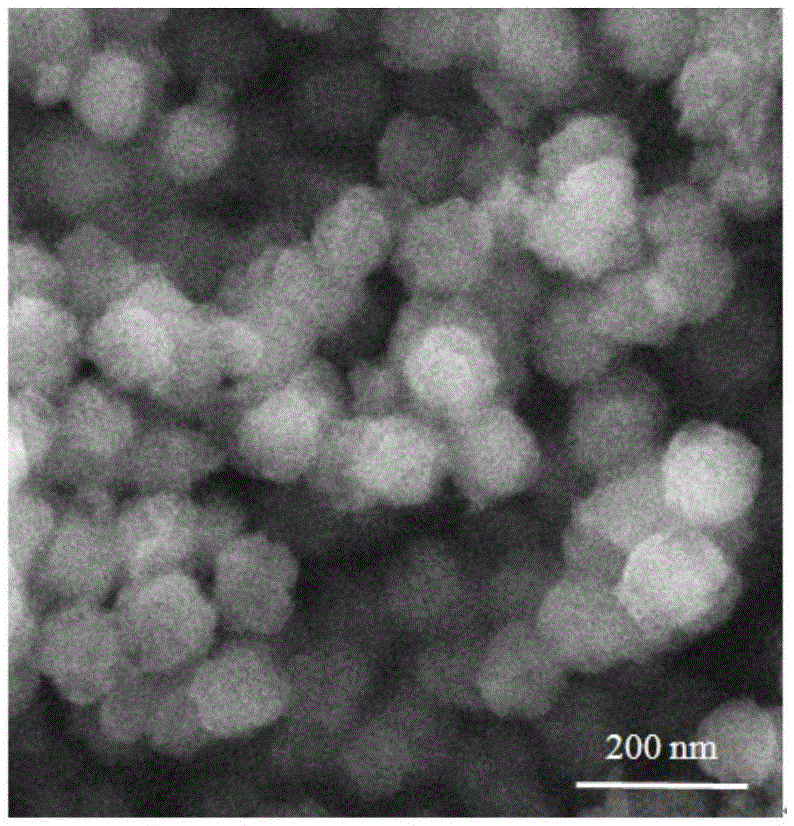
[0038] 0.2 mmol of triazine-based tricarboxylic acid and 0.8 mmol of zinc perchlorate were dissolved in 15 mL of DMF, sealed in a reaction kettle, and heated at 85° C. for three days to obtain triazine-based complex crystals. Single crystal diffraction analysis shows that the triazine-based complex has the chemical formula [Zn 3 L 2 (H 2 O) 3 ]·9H 2 O·8DMF, evenly distributed in the structure The open channel of , which belongs to 3D porous metal organic framework, its XRD analysis is shown in the appendix figure 1 , the XRD pattern shows characteristic absorptions at 2θ 5.6, 11.3, 16.9 and 21.3. Microscopic analysis of the triazine-based complex crystal shows that its crystal appearance is amorphous in water.
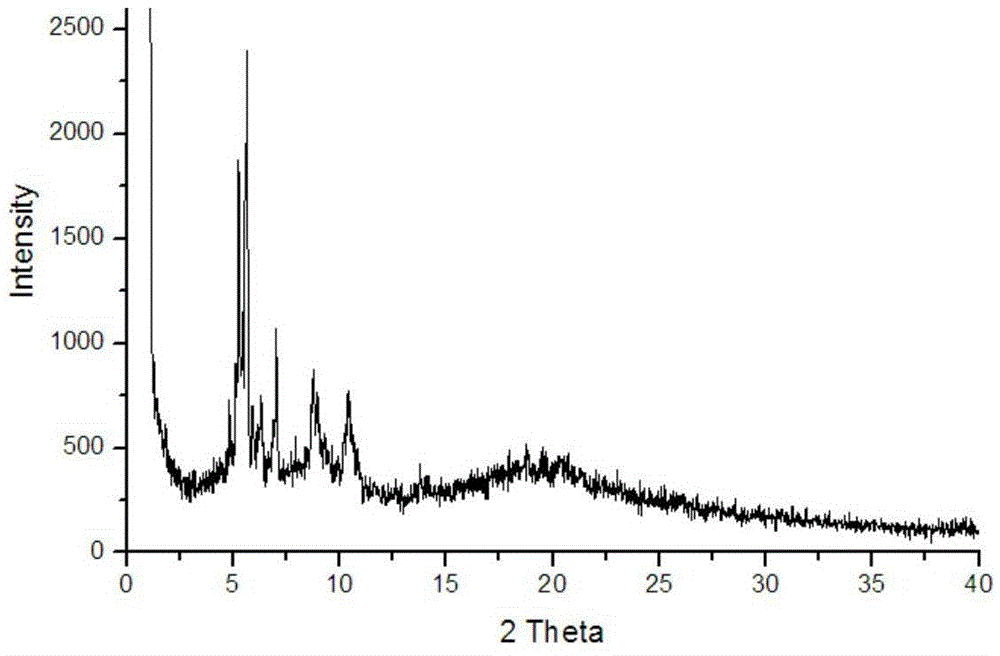
[0039] (1) Preparation of nano-triazine-based complexes:
[0040] Dissolve 0.8g of zinc nitrate in 1mL of water to prepare an aqueous solution of zinc nitrate, add this solution to 1mL o...
Embodiment 2
[0048] (1) Preparation of nano-triazine-based complexes:
[0049] Dissolve 1.0g of zinc nitrate in 1mL of water to prepare an aqueous solution of zinc nitrate, add this solution to 1mL of DMF solution containing 1.0g of triazine-based tricarboxylic acid, shake and blend, and carefully add to the upper layer of the mixture 1mL of absolute ethanol and 2mL of an aqueous solution of potassium hydroxide with a mass fraction of 7% were shaken and blended to obtain a colloidal homogeneous liquid, which was left to stand for 0.8h and then centrifuged, and the obtained solid was washed three times with DMF and centrifuged to prepare The nano-triazine-based complex was obtained with a yield of 83%.
[0050] (2) Preparation of triazine-based tricarboxylic acid H 3 L
[0051] Triazine-based tricarboxylic acid H in (1) 3 The preparation steps of L are as follows: under stirring conditions, blend 13mmol of 4-aminobenzoic acid and 15mL of an aqueous solution containing 15mmol of sodium hy...
Embodiment 3
[0057] (1) Preparation of nano-triazine-based complexes:
[0058] Dissolve 1.2g of zinc nitrate in 1mL of water to prepare an aqueous solution of zinc nitrate, add this solution to 1mL of DMF solution containing 1.1g of triazine-based tricarboxylic acid, shake and blend, and carefully add to the upper layer of the mixture 1mL of absolute ethanol and 1mL of an aqueous solution of potassium hydroxide with a mass fraction of 5% were shaken and blended to obtain a colloidal homogeneous liquid, which was left to stand for 0.8h and then centrifuged, and the obtained solid was washed three times with DMF and centrifuged to prepare The nano-triazine-based complex was obtained with a yield of 83%.
[0059] (2) Preparation of triazine-based tricarboxylic acid H 3 L
[0060] Triazine-based tricarboxylic acid H in (1) 3 The preparation steps of L are as follows: under stirring conditions, blend 13mmol of 4-aminobenzoic acid and 15mL of an aqueous solution containing 16mmol of sodium hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com