Method for screening daptomycin high-producing strain by adopting sodium glutamate tolerance model
A technology of sodium glutamate and daptomycin, applied in the field of biomedicine, to achieve the effect of wide source, high potency and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Mutagenesis: A single spore suspension of Streptomyces roseosporus was subjected to ultraviolet (UV) mutagenesis.
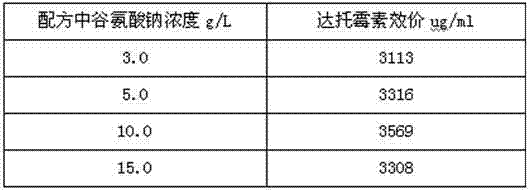
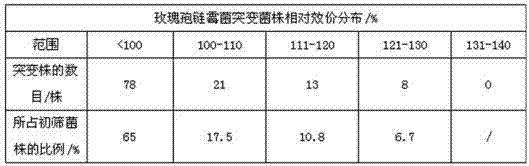
[0028] Primary screening: Prepare 2L of separation medium according to the following formula: soluble starch 20.0 g / L, magnesium sulfate 1.0 g / L, potassium nitrate 1.0 g / L, ferrous sulfate 0.01 g / L, sodium chloride 0.5 g / L, phosphoric acid Dipotassium hydrogen 0.5 g / L, sodium glutamate 150 g / L, agar 15.0 g / L, pH7.3. Prepare 50 sterile petri dishes, and pour the medium into the petri dishes after sterilization to form a flat plate. Make a monospore suspension from the mature slant of the starting strain with physiological saline, oscillate, disperse with glass beads for 15 minutes, and collect about 5ml of the monospore suspension by sterile filtration in a sterile petri dish (to make the final concentration of spores reach 10 6 -10 7 perml), UV (power 30W, lamp distance 35cm) irradiated for 60s. Appropriately dilute the single spore suspension after th...
Embodiment 2
[0039] Mutagenesis: The monospore suspension of Streptomyces roseosporus was mutagenized by ethyl methanesulfonate (EMS).
[0040] Primary screening: Prepare 2L of separation medium according to the following formula: soluble starch 20.0 g / L, magnesium sulfate 1.0 g / L, potassium nitrate 1.0 g / L, ferrous sulfate 0.01 g / L, sodium chloride 0.5 g / L, phosphoric acid Dipotassium hydrogen 0.5 g / L, sodium glutamate 200 g / L, agar 15.0 g / L, pH7.4. Prepare 60 sterile petri dishes, and pour the culture medium into the petri dishes after sterilization to form a flat plate. Use potassium phosphate buffer solution of pH 7.0 to make a single spore suspension from the mature slant of the starting strain, oscillate, disperse the glass beads for 15 minutes, and collect about 5 ml of the single spore suspension by sterile filtration (to make the final concentration of spores reach 10 6 —10 7 each / ml), add the alkylating agent EMS, the final concentration is 5%, shake for 45 minutes, add sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com