Ospemifene polymorphic substance
A polymorph, crystal form technology, applied in ether separation/purification, ether preparation, organic chemistry, etc., can solve problems such as unseen ospemifene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Ospemifene polymorph I
[0046] Add ospemifene sample (1g, 2.64mmol) in the reaction flask, acetonitrile (5mL) is heated up to 60 ℃, after the sample is completely dissolved, add water (2.5mL) dropwise, after stirring for 10min, slowly cool down to 25 ℃, in the Stirring was continued at high temperature for 30 min, and then filtered under reduced pressure to obtain a white solid.
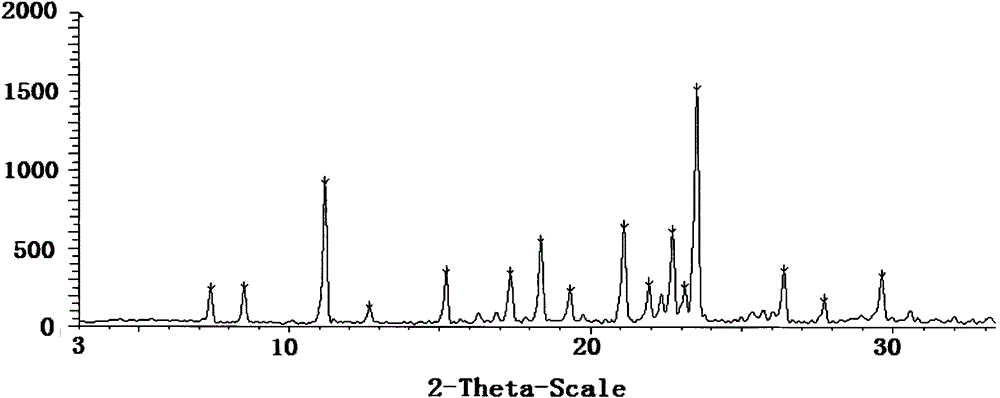
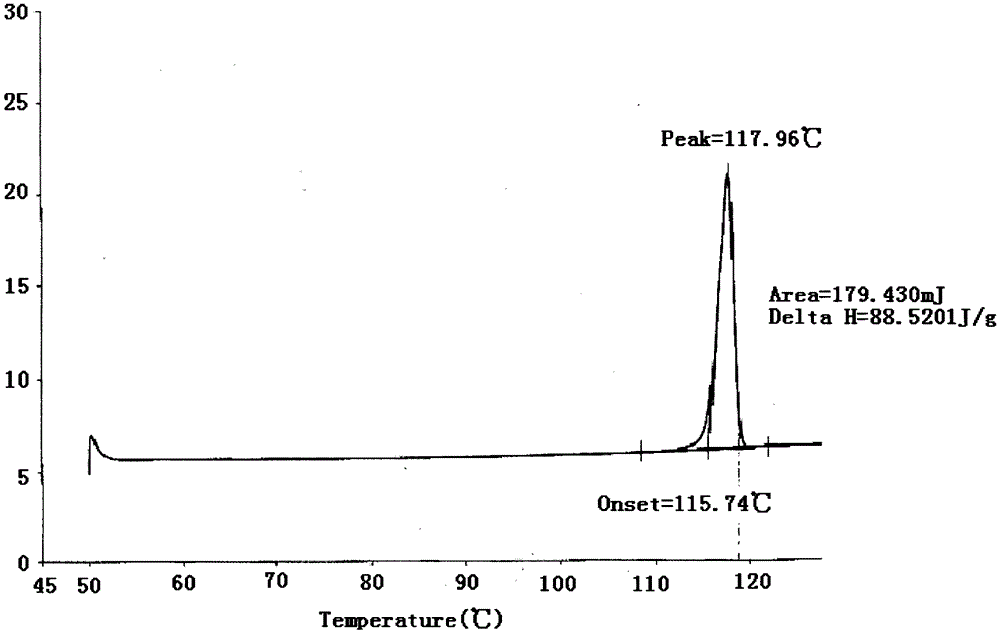
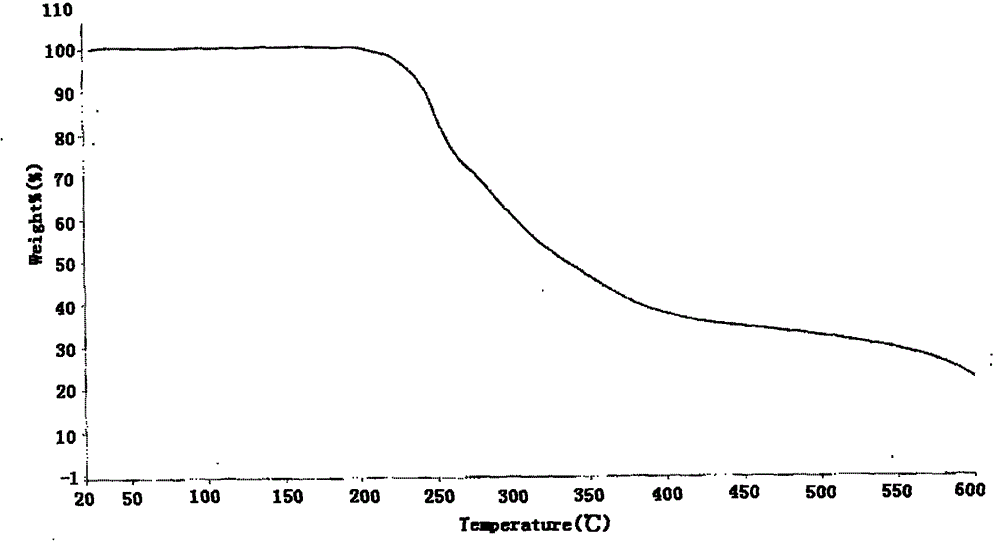
[0047] Form I is characterized by an X-ray powder diffraction pattern with peaks at the following approximate diffraction angles (2Θ): 11.2, 18.4, 21.1, 22.7, and 23.5. figure 1 An X-ray powder diffraction pattern of Form I is provided. figure 2 The Type I DSC curve shown in shows an endotherm onset at 116-118°C at a scan rate of 10°C / min. image 3 The Type I TGA curve shown indicates the onset of weight loss decomposition around 200°C at a scan rate of 20°C / min.
Embodiment 2
[0048] Embodiment 2: Ospemifene polymorphic form II
[0049] A sample of ospemifene (1 g, 2.64 mmol) was added to the reaction flask, and methanol (4 mL) was heated to 65 ° C. After the sample was completely dissolved, the temperature was slowly lowered to 30 ° C, and the organic solvent was concentrated to dryness to obtain a white solid.
[0050] Form II is characterized by an X-ray powder diffraction pattern with peaks at the following approximate diffraction angles (2Θ): 11.2, 17.3, 18.3, 22.7, and 23.5. Figure 4 An X-ray powder diffraction pattern of Form II is provided.
Embodiment 3
[0051] Embodiment 3: Ospemifene polymorphic form III
[0052] Add ospemifene sample (1g, 2.64mmol) into the reaction flask, heat up acetonitrile (2mL) to 65°C, and slowly cool down to 25°C after the sample is completely dissolved, let stand to precipitate a large amount of crystals, and filter under reduced pressure to obtain white solid.
[0053] Form III is characterized by an X-ray powder diffraction pattern with peaks at the following approximate diffraction angles (2Θ): 11.2, 18.4 and 21.1. Figure 5 An X-ray powder diffraction pattern of Form III is provided.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com