Pulmonary tuberculosis curative effect evaluation kit and its application
A curative effect evaluation and kit technology, which is applied in the field of pulmonary tuberculosis curative effect evaluation kits, can solve the problems of time-consuming, easy misdiagnosis, and poor discrimination efficacy, and achieve the effects of high efficiency, easy operation, high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the composition of pulmonary tuberculosis curative effect evaluation kit
[0041] In this embodiment, the tuberculosis curative effect evaluation kit includes microtiter plate, protein standard composition, biotin-labeled antibody composition, horseradish peroxidase-labeled avidin, diluent, washing solution, bottom reagent solution, stop solution, microtiter plate stickers.
[0042] The microtiter plates include: anti-ALB antibody-coated microtiter plates, anti-APOA antibody-coated microtiter plates, anti-C3 antibody-coated microtiter plates, and the above microtiter plates were purchased from Abcam Aimei Jie Technology Co., Ltd. . Also included are anti-ARHGDIB antibody-coated microtiter plates and anti-FCN2 antibody-coated microtiter plates, which were purchased from Wuhan Huamei Bioengineering Co., Ltd.
[0043]In this embodiment, the protein standard composition includes: ALB, APOA, ARHGDIB, C3 and FCN2 five protein standards. Among them, the ALB, A...
Embodiment 2
[0060] Example 2: Establishment of the Logistic stepwise regression model of the pulmonary tuberculosis curative effect evaluation kit
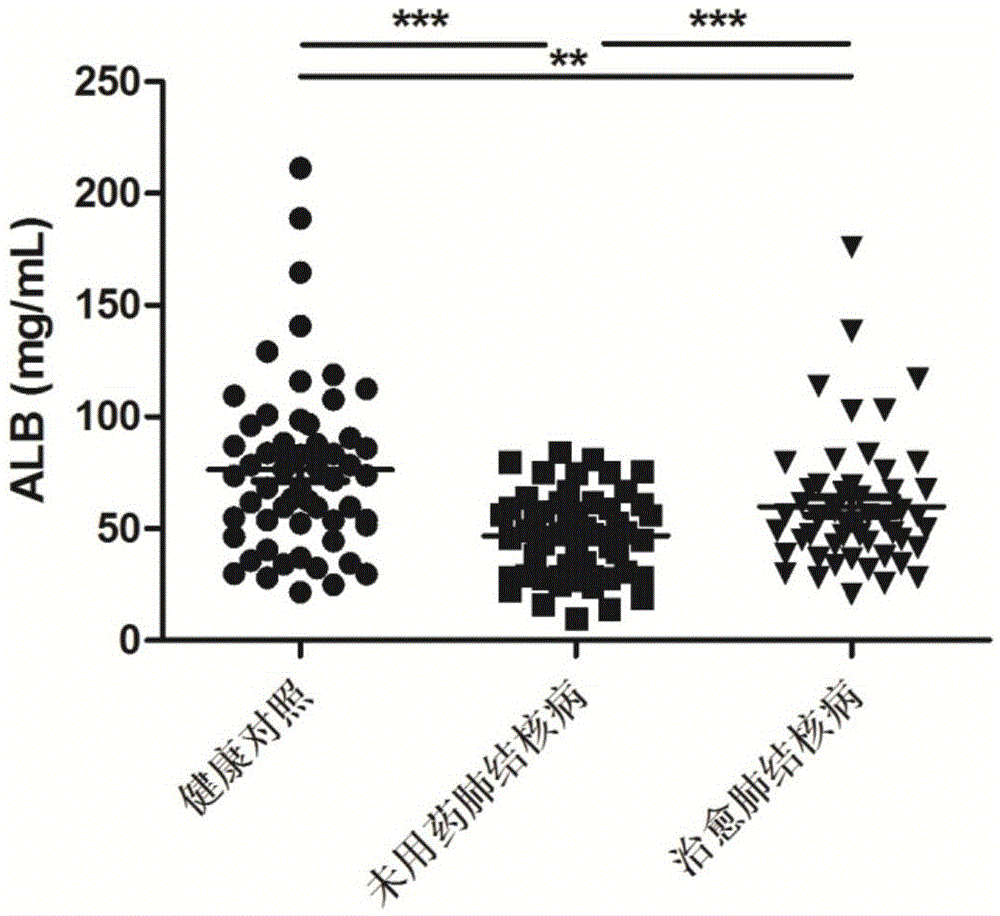
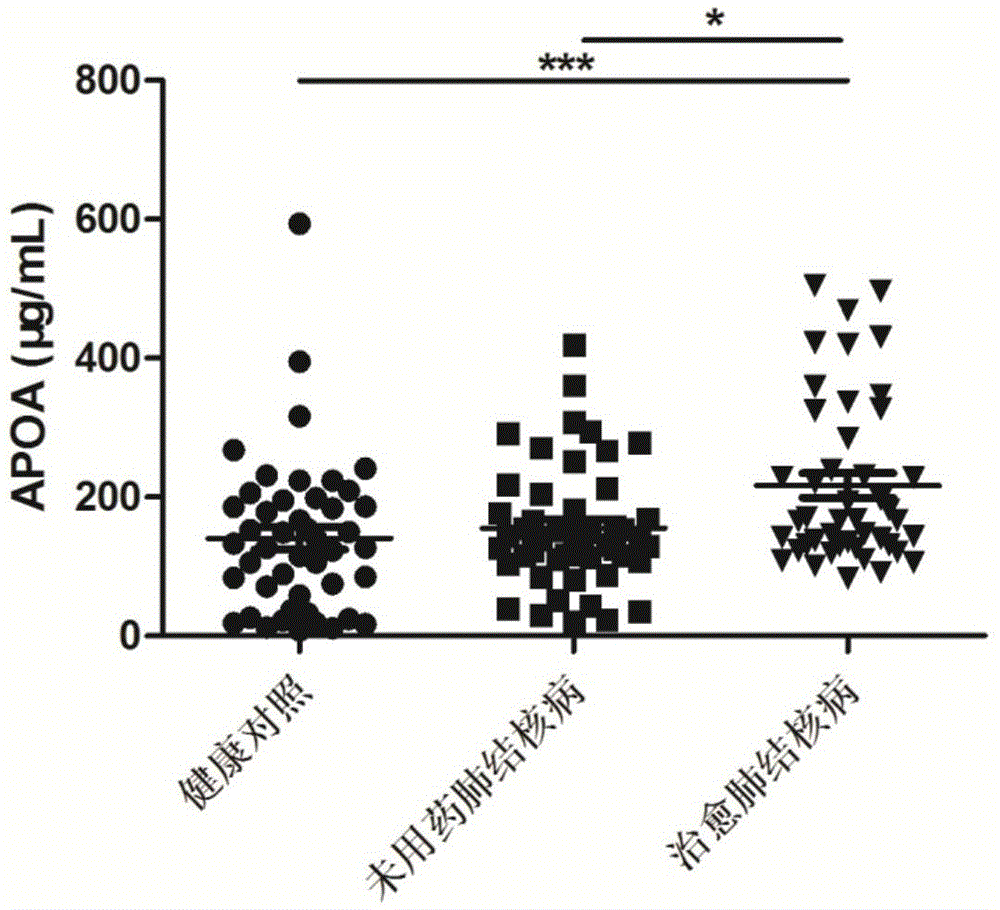
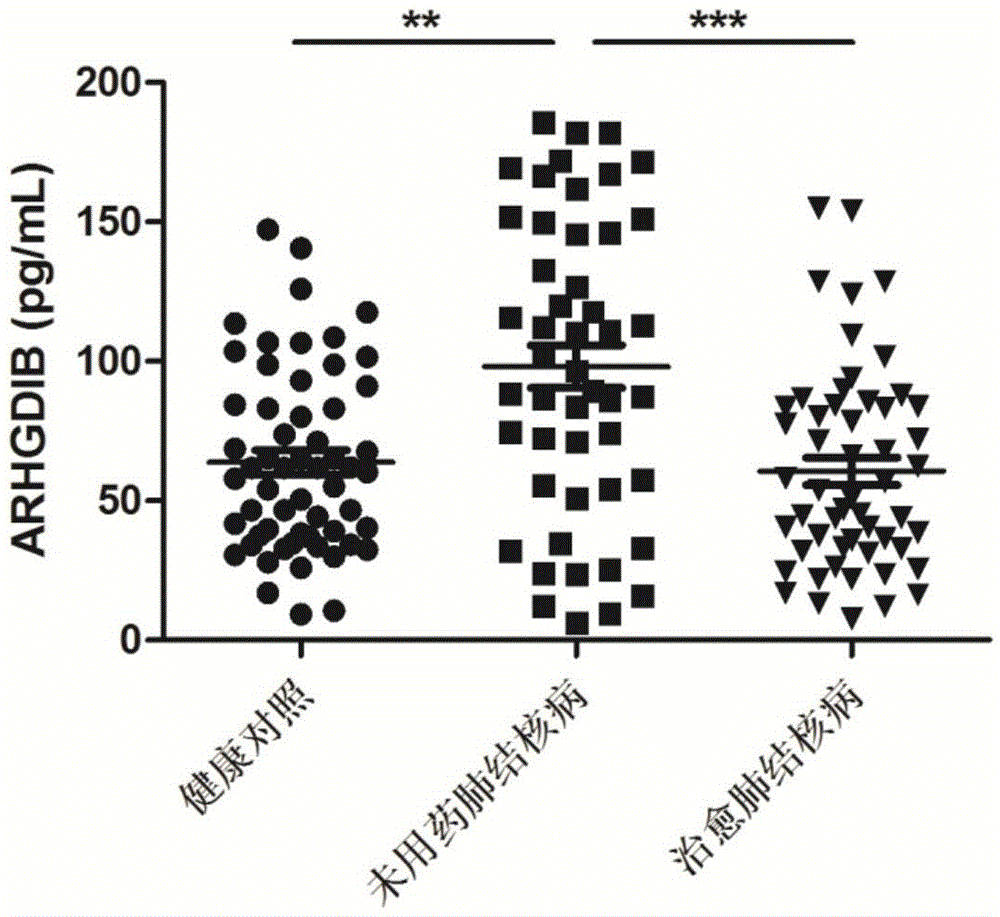
[0061] Tuberculosis efficacy evaluation kit was used to detect the expression levels of ALB, APOA, ARHGDIB, C3 and FCN2 proteins in the serum of untreated tuberculosis patients, cured tuberculosis patients, and healthy controls.
[0062] (1) Sample collection:
[0063] According to the diagnostic criteria of tuberculosis issued by the Ministry of Health of China, 57 cases of tuberculosis patients who did not take drugs were collected, and 59 cases of tuberculosis patients were cured. During the same period, 60 cases of healthy controls were collected. All HIV tests were negative, no hepatitis B, no diabetes, no asthma, no history of tuberculosis, normal liver and kidney function, no other congenital diseases, and other diseases such as chronic inflammation and autoimmune diseases were excluded. There was no other operation history, and there...
Embodiment 3
[0084] Example 3: Verification and application of the detection effect of the pulmonary tuberculosis curative effect evaluation kit
[0085] Leave-one-out cross-validation: take serum samples from cured tuberculosis patients, drug-naïve tuberculosis patients, and normal controls, and the sample collection and dilution methods are the same as those described in Example 2.
[0086] Add 0.1 mL of the diluted serum to be tested to the microtiter plate coated with anti-ALB antibody, anti-APOA antibody, anti-ARHGDIB antibody, anti-C3 antibody and anti-FCN2 antibody, and incubate at 37°C for 1 hour.
[0087] Pat the microtiter plate dry, add 0.1 mL of freshly diluted biotin-labeled antibody to each reaction well corresponding to the antibody coated on the microtiter plate, incubate at 37°C for 1 hour, and wash with washing solution.
[0088] Add 0.1 mL of freshly diluted horseradish peroxidase-labeled avidin to each reaction well, incubate at 37°C for 0.5-1 hour, and wash with washin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com