A leptin active peptide with a mutation in the c-helix region, its coding gene and application
A technology that encodes genes and helical regions, applied in the fields of biochemistry and molecular biology, can solve the problems of ineffective weight loss and difficulty in obtaining weight loss with wild-type human leptin protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] 1. Materials and methods
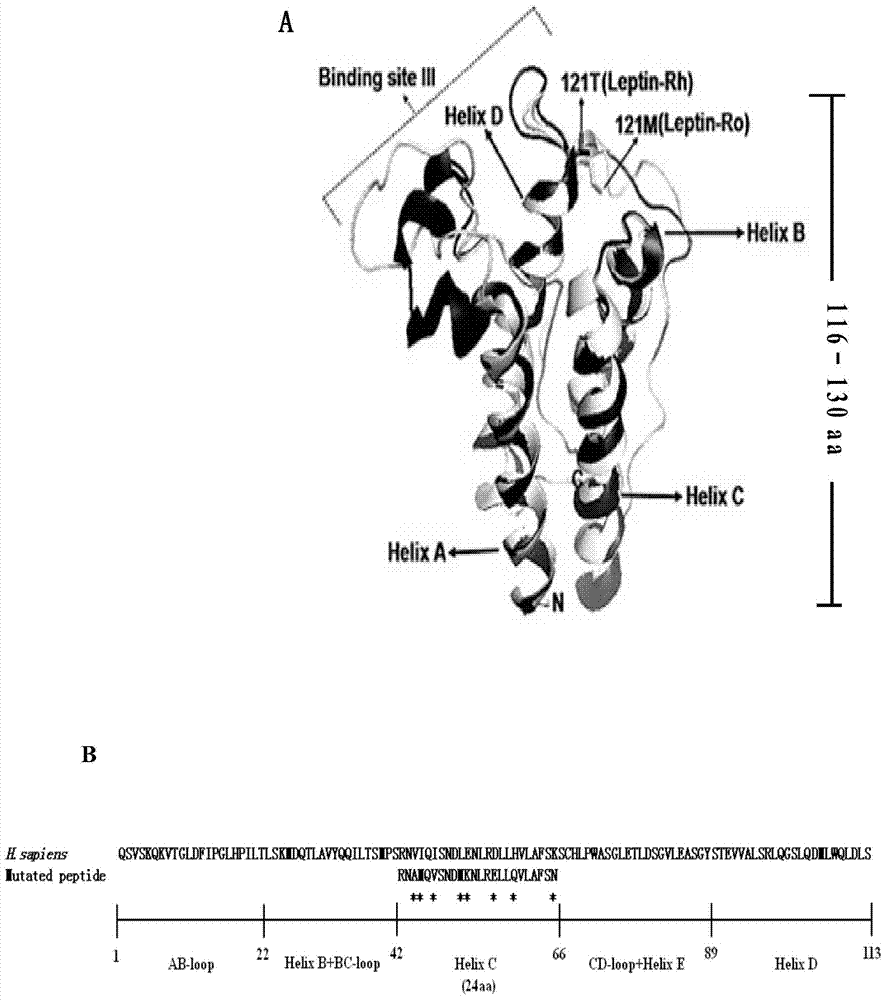
[0014] 1.1 Design and synthesis of leptin active peptide with mutant C-helical region
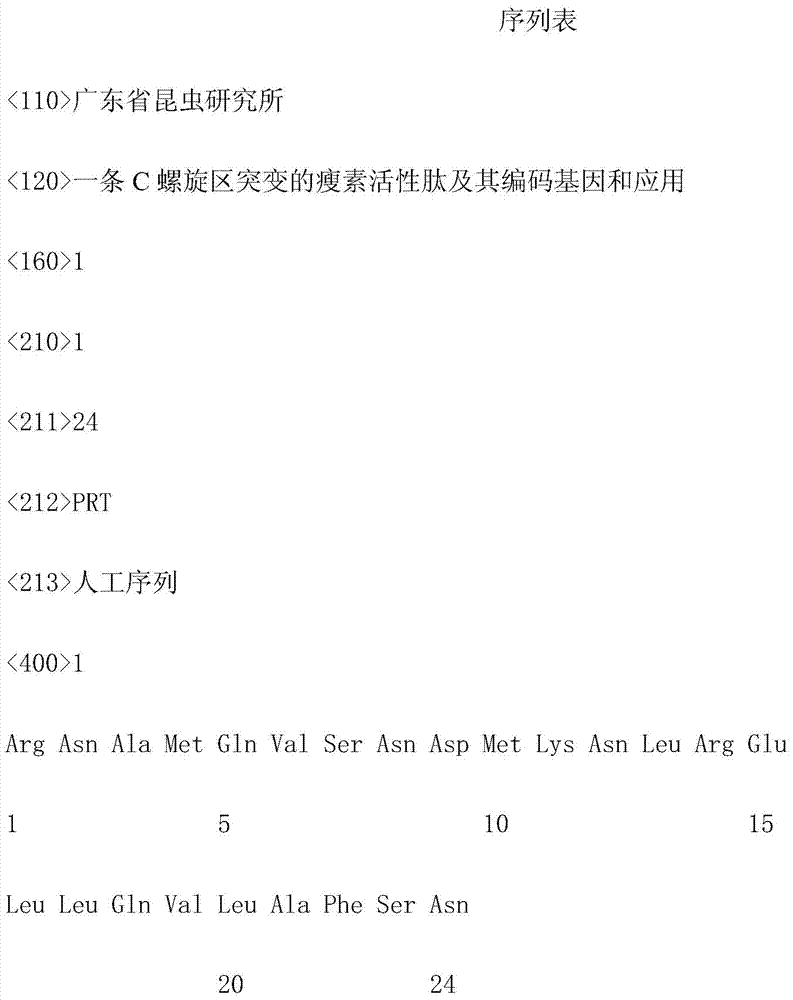
[0015] Using the amino acid sequence of human Leptin as a template, a leptin active peptide with a mutation in the C-helix region is designed in its functional region according to the changes in the hydrophilic / hydrophobic properties of the mutation site. shown). The leptin active peptide mutated in the C-helix region was synthesized by Shanghai Sangon Bioengineering Co., Ltd., and the concentration of the chemically synthesized leptin active peptide mutated in the C-helix region was >97.78%, which met the requirements for subsequent activity analysis. The accuracy of the synthesized leptin active peptide mutated in the C-helical region was detected by mass spectrometry, and its amino acid sequence was confirmed to be RNAMQVSNDMKNLRELLQVLAFSN (specifically shown in SEQ ID NO.1).
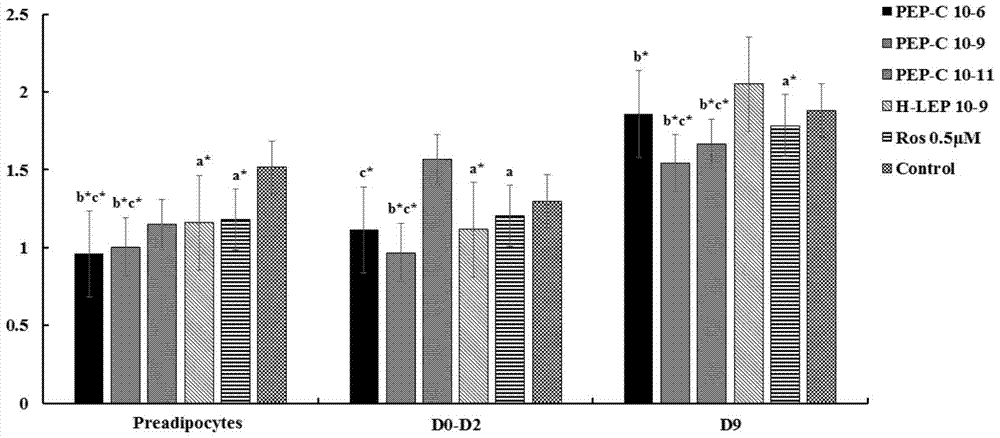
[0016] 1.2 In vitro lipid-lowering activity analysis of leptin active pepti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com