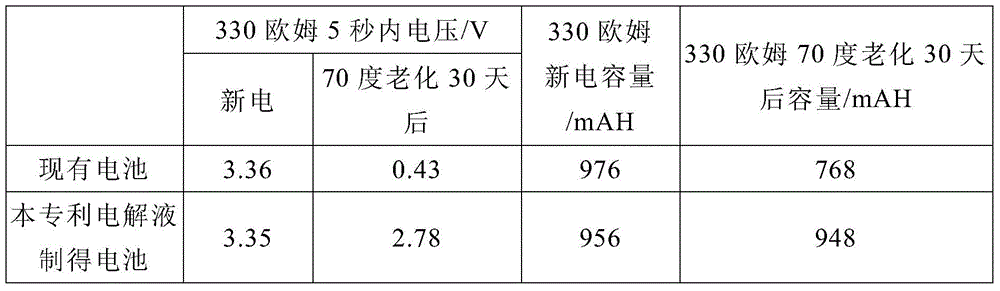
A kind of lithium thionyl chloride battery electrolyte preparation method
A technology for preparing lithium thionyl chloride batteries and electrolytes, which can be applied to electrolytes, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of waste, high cost, and many operation steps, and can reduce consumption or blackening degree and reaction time. Short, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take 580g of lithium salt LiAlCl at a dew point of -36°C 4 (lithium salt LiAlCl 4 From LiCl and AlCl 3 Made by melting, the molar ratio of LiCl to AlCl 3 Molar excess of 2%) was added into a 3L round bottom flask, and then 280g of sulfur dioxide was passed into the flask, the purity of sulfur dioxide gas was ≥99.99%, the product was left to stand for 10h, and then the distillate of 4250g of thionyl chloride was slowly added in three times In the round-bottomed flask, the mass ratio of the distillate of thionyl chloride to the lithium salt after the reaction is 5:1, shake well and dissolve, then add 2 lithium slices with a thickness of 0.25mm and a length of 15cm, with a quality of 1.4g (the amount added is lithium Salt LiAlCl 4 0.24% of the mass), and leave it to get the electrolyte.
Embodiment 2
[0020] Take 580g of lithium salt LiAlCl at a dew point of -38°C 4 (lithium salt LiAlCl 4 From LiCl and AlCl 3 Made by melting, the molar ratio of LiCl to AlCl 3 Molar excess of 5%) was added into a 3L round bottom flask, and then 232g of sulfur dioxide was passed into the flask, the purity of sulfur dioxide gas was ≥99.99%, the product was left to stand for 5h, and then the distillate of 2840g of thionyl chloride was slowly added in three times In the round bottom flask, the mass ratio of the distillate of thionyl chloride to the lithium salt after reaction is 3.5:1, shake well and dissolve, then add 2 pieces of 0.25mm thick lithium slices, the quality is 1.45g (the amount added is lithium salt LiAlCl 4 0.25% of the mass), and leave it to get the electrolyte.
Embodiment 3
[0022] Take 580g of lithium salt LiAlCl at a dew point of -36°C 4 (lithium salt LiAlCl 4 From LiCl and AlCl 3 Made by melting, the molar ratio of LiCl to AlCl 3 Molar excess of 3%) was added into a 3L round bottom flask, and then 290g of sulfur dioxide was introduced into the flask, the purity of sulfur dioxide gas was ≥99.99%. In the round bottom flask, the mass ratio of the distillate of thionyl chloride to the lithium salt after reaction is 5:1, shake well and dissolve, then add 2 pieces of 0.25mm thick lithium slices, the quality is 0.87g (the amount added is lithium salt LiAlCl 4 0.15% of the mass), and leave it to get the electrolyte.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com