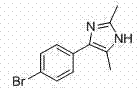
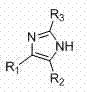
Preparation method of 2,4,5-trisubstituted imidazole compound
A compound, a technology for imidazole, which is applied in the field of preparation of imidazole compounds, can solve the problems of poor applicability of different functional groups, difficult to obtain raw materials, low product yield and the like, and achieves the effects of easy operation of steps, cheap reaction raw materials and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of 5-methyl-4-phenyl-1H-imidazole
[0049] 1. Preparation of raw material 2-methyl-2-nitro-3-phenyloxirane
[0050] Add 2.1g (20mmol) of benzaldehyde and 9.0ml (100mmol) of nitroethane into a three-necked flask, and slowly add Et 3N 0.3ml (2mmol). After the addition was complete, stir at room temperature under nitrogen protection for 18h. After the disappearance of raw materials detected by the TLC plate, the excess solvent was evaporated under reduced pressure, and the crude product α-nitroalcohol was directly dissolved in dichloromethane, and then 7.4 mL (42 mmol) of N,N-diisopropylethylamine and formaldehyde were added sequentially. Sulfonyl chloride 1.9 mL (24 mmol), after the addition, the reaction gradually returned to room temperature. After the TLC plate detected that the raw materials disappeared, the reaction system was extracted with dichloromethane, and the organic phase was washed twice with 10ml of 2M dilute hydrochloric acid and o...
Embodiment 2
[0075] Example 2, 5-ethyl-4-phenyl-1 H- Preparation of imidazole
[0076] Replace 2-methyl-2-nitro-3-phenyloxirane with 2-ethyl-2-nitro-3-phenyloxirane, the molar weight remains the same, and the rest are the same as in Example 1. 5-Ethyl-4-phenyl-1 was obtained as a white solid H- Imidazole 163mg, yield 95%. Its structural formula is:
[0077]
[0078] 1 H NMR (500 MHz, DMSO) δ 7.61 (s, 1H), 7.58 (dd, J = 8.1, 1.0 Hz, 2H), 7.39 (dd, J = 10.7, 4.9 Hz, 2H), 7.25-7.19 (m, 1H), 2.76 (q, J = 7.5 Hz, 2H), 1.22 (t, J = 7.5 Hz, 3H). 13 C NMR (125 MHz, DMSO) δ 134.78, 134.52, 128.88, 126.60, 126.19, 19.49, 14.61. HRMS (ESI): m / z calcd for C 11 h 13 N 2 [M+H] + :173.1073, found: 173.1073.
Embodiment 3
[0079] Example 3, 4-(4-chlorophenyl)-5-methyl-1 H - Preparation of imidazole
[0080] Replace 2-methyl-2-nitro-3-phenyloxirane with 3-(4-chlorophenyl)-2-methyl-2-nitrooxirane, the molar weight remains unchanged, and the rest With embodiment 1. The product 4-(4-chlorophenyl)-5-methyl-1 was obtained as a yellow solid H -Imidazole 171 mg, yield 89%. Its structural formula is:
[0081]
[0082] 1 H NMR (500 MHz, DMSO) δ 12.11 (s, 1H), 7.64 (d, J = 8.4 Hz, 2H), 7.60 (s, 1H), 7.43 (d,J = 8.4 Hz, 2H), 2.38 (s, 3H). 13 C NMR (125 MHz, DMSO) δ 134.40, 130.36, 128.80, 127.78, 12.21. HRMS (ESI): m / z calcd for C 10 h 10 ClN 2 [M+H] + :193.0527, found: 193.0537.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com